Abstract
The ionic liquid analogue containing MgCl2 based on choline chloride and glycerol was reported. The solubility of MgCl2 in the ionic liquid analogue based on choline chloride and glycerol was measured from T = 293–393 K. The empirical equation about the solubility and temperature was obtained. Thermal analysis showed that the ionic liquid analogue was stable from room temperature to 140 °C. The physical properties such as conductivity σ, density ρ and viscosity η of ionic liquid analogue were measured as function of the content of MgCl2 and temperature. An empirical equation about the density (ρ) and temperature was obtained. The ions transport behaviours are analyzed using hole-theory. It is shown that the conductivity of the ionic liquid analogues is controlled by the ion mobility and the suitable voids.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As green solvents, ionic liquids especially air and water stable ionic liquids have gained great attention [1–3]. In recent decades, the study of ionic liquids is focused on the chemical reaction and separation processes. Some researchers studied the thermal properties of ionic liquids [4–6], however, the physical and chemical properties is few reported which is the foundation for its use in chemical synthesis, electrochemistry and other fields.
Ionic liquids based on choline chloride by the advantage of non-toxicity, biocompatibility, simple preparation, air and water stability at room temperature may use widely [7]. Figueiredo [8] reported the differential capacity of a deep eutectic solvent based on choline chloride and glycerol. The use of ionic liquids based on choline chloride and urea for metal deposition have been reported [9, 10]. Our team has reported the structure and ions transport behaviour of the ionic liquid based on choline chloride and ethylene glycol [11].
Magnesium is used widely in electronics, aero-space, electronic communication, national defence and military as its low density, high strength, electromagnetic shielding, and excellent casting and mechanical processing properties. Many researchers focus on the study of ionic liquids containing magnesium salts. Nobuko Yoshimoto [12] reported a novel polymeric gel electrolyte systems containing magnesium salt with ionic liquid. NuLi [13] electrodeposited magnesium film from BMIMBF4 ionic liquid. Pandey and Hashmi [14] Studies on a novel magnesium ion conducting gel polymer electrolyte based on a room temperature ionic liquid. The previous studies mainly used organic magnesium salt, such as Mg(ClO4)2, Mg(CF3SO3)2, Mg[(CF3SO2)2N]2 and Grignard reagent, and there is almost no report about ionic liquids containing inorganic magnesium salt.
Our previous results show that when the mass fraction of choline chloride is 48%, the ionic liquid analogue based on choline chloride and glycerol obtained the minimum viscosity and maximum conductivity (Fig. 1). In this article, we studied the solubility of anhydrous magnesium chloride in such an ionic liquid analogue at different temperature. The viscosity, conductivity and density were measured as a function of temperature and compositions.
Experimental section
Materials
Choline chloride (ChCl) (AR, w > 0.99, Sinopharm Chemical Reagent Co., Ltd, China) was recrystallized from ethanol absolute, filtered and dried under vacuum. Glycerol (Gl) (AR, w > 0.99, Tianjin Baishi Chemical Co., Ltd, China) was used as received. Anhydrous magnesium chloride (MgCl2) (AR, w > 0.99, Tianjin Kermel Chemical Reagent Co., Ltd, China) was used as received.
Property measurements
The solubility of choline chloride in glycerol
The solubility of anhydrous magnesium chloride in the ionic liquid analogue was measured at 293, 313, 333, 353, 373 and 393 K, respectively. At different temperatures, anhydrous magnesium chloride was gradually added into a certain amount of ionic liquid analogue until choline chloride was not dissolved and formed a homogeneous and colourless liquid. The solubility of anhydrous magnesium chloride in the ionic liquid analogue was confirmed. The oil bath was used to control the temperature. The uncertainty for the temperature measurement was estimated to be ±1 K. The overall uncertainty for the solubility measurement was estimated to be 0.0001 (molar fraction).
Water content measurement
The water content measurement is confirmed by Karl Fischer Analyzer (Labindustries, INC, USA) in open air. The titer value of Karl Fischer regent is measured by titrating pure water. The molar concentration of MgCl2 is 0.122 mol L−1.
Thermal analysis experiment
Thermal analysis (TG) is determined by a thermal analysis instrument (SDT-Q600, TA Instruments, USA) from room temperature to 300 °C under the protection of nitrogen atmosphere at a heating rate of 1 °C min−1.
The conductivity measurement
The conductivity was measured using conductivity instrument (YSI 3200.USA) at the temperature ranging from 293 to 393 K. The platinum black electrode was used as the conductance electrode. As conductivity was measured, the platinum black electrode was submerged in the sample whose temperature was controlled by a precision thermometer with an uncertainty of ±0.01 K. Anhydrous magnesium chloride was gradually added into a certain amount of ionic liquid analogue. When anhydrous magnesium chloride completely dissolved in the ionic liquid analogue, conductivity was measured. The changes of conductivity as a function of temperature were also measured. The uncertainty in experimental measurements is about 0.001 mS cm−1.
The viscosity measurement
The viscosity of ionic liquid analogue was measured by rotary viscosimeter (NDJ-5S, Shan Hai Precision Scientific Instrument Co., Ltd, China) at the temperature ranging from 293 to 393 K. Anhydrous magnesium chloride was gradually added into a certain amount of ionic liquid analogue. When anhydrous magnesium chloride completely dissolved in the ionic liquid analogue, viscosity was measured. The changes of viscosity were also measured as a function of temperature. The uncertainty in experimental measurements is 0.01 mP s.
The density measurement
The density was measured by densimeter at the temperature ranging from 293 to 393 K. Anhydrous magnesium chloride was gradually added into a certain amount of ionic liquid analogue. When anhydrous magnesium chloride completely dissolved in the ionic liquid analogue, density was measured. The changes of density were also measured as a function of temperature. The repeatability and uncertainty in experimental measurements have been found to be lower than 2 × 10−5 g cm−3 for the density.
Results and discussion
The solubility of MgCl2 in ionic liquid analogue
Figure 1 shows when the mass fraction of choline chloride is 48%, the ionic liquid analogue based on choline chloride and glycerol obtained the minimum viscosity and maximum conductivity. In order to obtain minimum viscosity and maximum conductivity, we chose this ratio to synthesized ionic liquid analogue and added MgCl2 to such an ionic liquid analogue and measured the solubility.
Table 1 shows that the solubility of MgCl2 in ionic liquid analogue increases with the increasing temperature.
According to the theory of solid–liquid phase equilibrium thermodynamics, in a limited temperature range, the ideal solubility is expressed as Eq. 1 [15]
where x is the solubility of MgCl2 (molar fraction), T is absolute temperature (K), R is gas constant (J mol−1 K−1), T m is fusion temperature of MgCl2 (K) and \( \Updelta S^{\text{f}} \) is melting entropy of MgCl2 (J mol−1 K−1).
Therefore, in a certain temperature range, the relationship between solubility and temperature can be simplified as Eq. 2
where A and B are model parameters.
According to Fig. 2, we can get the tie-in Eq. 3
The measurement of water content
The synthetic process of ionic liquid analogue is in the air and may absorb moisture, so the water content is determined using Karl Fischer Analyzer. The titer value of Karl Fischer regent can be described by Eq. 4:
where F is the titer value of Karl Fischer regent (mg mL−1), W 1 is the mass of pure water (mg) and V 1 is the volume of Karl Fischer regent (mL). According to Eq. 4, it can be calculated that the F is 2.1472 mg mL−1. The water content of ionic liquid analogue can be described by Eq. 5:
where F is the titer value of Karl Fischer regent (mg mL−1), W 2 is the mass of ionic liquid analogue (mg), and V 2 is the volume of Karl Fischer regent (mL). According to Eq. 5, it can be calculated that the water content of ionic liquid analogue solution is 0.0208 wt% (208 ppm).
In general, when water content less than 0.2 wt%, the solution can be viewed as water free. From above analysis, it can be noted that the water of the ionic liquid analogue is water free.
Thermal stability analysis
The most significant characteristics of ionic liquids are their low melting points and extremely large liquids range. The thermal stability analysis of MgCl2–EG–ChCl is illustrated in Fig. 3.
From Fig. 3, it is noted that there is almost no mass loss below 140 °C which indicates the ionic liquid analogue is stable from room temperature to 140 °C. At the same temperature range, the liquid range of the system is larger than that of the ionic liquid analogue based on choline chloride and ethylene glycerol [11]. This attributes to that glycerol with its three OH is a three dimensionally hydrogen-bonded liquid and can form stronger hydrogen bonding than ethylene glycerol [16].
Viscosity
One of the main differences between ionic liquids and aqueous solutions is the comparatively higher viscosity of the former. In many applications, low viscosity of ionic liquid is expected, especially in electrochemical applications.
The experimental viscosity (η) data for Gl–ChCl–MgCl2 as a function of temperature is shown in Fig. 4. The molar concentration of MgCl2 is about 0.122 mol L−1.
Figure 4 shows that the viscosity covers the range from 30 to 700 mP s. It is noticeable that the viscosity of Gl–ChCl–MgCl2 decreases with the increasing temperature. This results from the weakening of Van Der Waals Force and hydrogen bonding between different groups with the increasing temperature. The reduced resistance allows ions moves easily. It is also noted that the viscosity of Gl–ChCl–MgCl2 decreases significantly from 293 to 353 K and decreases slowly from 353 to 393 K. This attributed to that the molecule obtains enough kinetic energy to overcome intermolecular force at 353 K. This temperature can ensure the free movement of molecules. When the temperature continues to rise, the weakening of intermolecular forces is not so significant.
In electrochemical applications, low viscosity and high conductivity are expected at relatively low temperature. The reported electrochemical applications of ionic liquid is mainly in the range of 343–353 K. So in the following, we mainly studied the physical properties at about 353 K.
Table 2 shows the viscosity increases with the increasing content of MgCl2. When more MgCl2 are added, the number of molecules in per unit volume increases which lead to the Van Der Waals Force between different groups become stronger. It also can be concluded that maybe MgCl2 plays the role of bridge that connect the other ionic groups. As more MgCl2 are added, more bridges are built. Then the network between different groups is formed. Since the formation of network structure, viscosity increases.
The hole-theory was developed for molten salts but has been shown to be very useful for ionic liquids. It is shown that the value of E η is related to the size of the ions and the size of the voids present in the liquid. It has been shown that hole-theory can be applied to both ionic liquid and molecular fluids to account for viscosity.
The changes in viscosity (η) with temperature can be described by Eq. 6 [17].
where η0 is a constant and E η is the energy for activation of viscous flow. E η is the energy for activation of viscous flow.
Figure 5 shows that all of the data obey Eq. 6 well. The E η is larger than that of the ionic liquid analogue based on choline chloride and ethylene glycol (5.3–5.8 kJ mol−1) [10]. The hydrogen bond of glycerol is greater than ethylene glycol. The intermolecular force of glycerol is stronger than that of ethylene glycol. In order to move easily, glycerol molecule needs greater energy for activation of viscous flow.
It has been shown empirically that E η values are related to the melting point of liquids (T m) by Eq. 7,
This has been found to be valid for a range of molecular liquids and high-temperature molten salts. According to Eq. 7, the calculated value of T m is about 988 °C, which is significantly bigger than the experimental data at about 20 °C. This suggests that the empirical relationship observed in Eq. 7 for molecular liquids and high temperature molten salts, is not valid for room temperature ionic liquids analogues and may result from the large ionic size found in the ionic liquids analogues.
Conductivity
From Fig. 6, it is noticeable that the conductivity of ionic liquid analogue increases with the increasing temperature. The conductivity covers the range from 1 to 20 mS cm−1. The intermolecular forces become weak with the increasing temperature. Also with the increasing temperature, the ions obtain more kinetic energy to overcome the intermolecular force and move easily.
Compared Fig. 4 with Fig. 6, it is found that the conductivity increases but the viscosity decreases in the same temperature range. This may be the results from the high viscosity that prevents the movement of ions.
Table 3 shows that the conductivity decreases as the increasing content of MgCl2. It can be concluded that MgCl2 plays the role of bridge that connects other ionic groups in ionic liquid analogue. As more MgCl2 are added, more bridges are built. Then the network between different groups is formed. The formation of network structure limits the movements of ions.
Compared Table 2 with Table 3, it can be noted that the changes of conductivity also relates to the changes of viscosity. The conductivity decreases with the increasing viscosity.
Similar to the viscosity data, conductivity of ionic liquids analogues have been fitted to Eq. 8:
where E Λ is the activation energy for conduction.
Figure 7 shows the data for all of the ionic liquid analogues fit Eq. 8 accurately. The values of E Λ is 30.305 kJ mol−1. Figure 7 shows a good linear correlation between ln σ and the reciprocal of T as a function of temperature, which shows that the ionic mobility is controlling the conductivity of the ionic liquid analogues. Since the slope is approximately constant, it also shows that the identity of charge carriers remains approximately constant. It is therefore evident that it is not the number of charge carriers that control the conductivity, but rather their mobility. This also reinforces the idea that the limitation for ionic movement is the availability of voids of suitable dimensions and charge transport is governed by the density of voids but not the density of charged species.
For ionic liquids the conductivity is generally governed by the mobility of the charge carrier. Hence, a plot of σ versus η−1 should be a linear for a given charge carrier.
Figure 8 shows the correlation between σ and η−1 as a function of temperature. If there is a kind of species that control charge carrier, the slope of the system would be expected to be constant. From Fig. 8, it can be noted a poorly linear relationship for the system which indicates that the charge carriers are dominated by more than one species.
Density
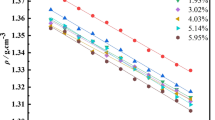
Table 4 shows that the density of ionic liquid analogue increases with the increasing content of MgCl2.
The temperature dependence of density (ρ) for the ionic liquid analogue was obtained. In general, in a narrow range of temperature, density ρ (g cm−3) is expressed as Eq. 9 [18]
where parameters a and b are related to the coefficient of volume expansion (g cm−3 K−1) and extrapolated density at 0 K (g cm−3), respectively. T is temperature (K). The variation of density with temperature is shown in Fig. 9.
Figure 9 shows that the behaviour of density as a function of temperature is linear as expected. All the data fitted Eq. 9 well and the fit coefficient is 0.996. An empirical equation was obtained as follows:
The coefficient of thermal expansion of IL studied is defined by the following equation:
where α is the coefficient of thermal expansion, V is the volume of the IL and ρ is the density of the IL. The value of α obtained from the slope of the linear fit is 3.5343 × 10−4 K−1.
The molar volume V m can be calculated by Eq. 12
where M is the molar mass which is taken as the molar mass of the three components multiplied by their mole fraction in the mixture and ρ is the measured value of ionic liquid analogue. The calculated values of V m are shown in Table 5.
Conclusions
In this article, the ionic liquid analogue containing MgCl2 based on choline chloride and glycerol was synthesized. The solubility of MgCl2 in ionic liquid analogue was obtained at different temperatures. The empirical equation about the relationship between solubility and temperature was obtained. The ionic liquid analogue is stable from room temperature to 140 °C. The reasons for the changes of conductivity, viscosity and density as a function of temperature and compositions were explained. MgCl2 may play the role of bridge that connects the other ionic groups in ionic liquid analogue. As more MgCl2 are added, more bridges are built. Then the network between different groups is formed. Since the formation of network structure, viscosity increases and conductivity decreases. The hole-theory analysis confirms that not the density of charge carriers but the mobility of charge carriers and the available voids control the charge transport. The data and variation tendency of conductivity, viscosity and density as function of temperature and composition can provide theoretical basis for the structural research and functional ionic liquids design.
References
Endres F, Abedinw SZE. Air and water stable ionic liquids in physical chemistry. Phys Chem Chem Phys. 2006;8:2101–16.
Katase T, Onishi T, Imashuku S, Murase K, Hirto T, Awakura Y. Water content and properties aliphatic ammonium imide-type room temperature ionic liquid containing metals ions. Electrochemistry. 2005;73:686–94.
Abbott AP, Capper G, Davies DL, Munro HL, Rasheed RK, Tambyrajah V. Preparation of novel, moisture-stable, Lewis-acidic ionic liquids containing quaternary ammonium salts with functional side chains. Chem Commun. 2001;2010–2011.
Zhang ZH, Cui T, Zhang JL, Xiong H, Li JP, Sun LX, Xu F, Cao Z, Li F, Zhao JJ. Thermodynamic investigation of room temperature ionic liquid. J Therm Anal Calorim. 2010;101:1143–8.
Amarasekara AS, Owereh OS. Thermal properties of sulfonic acid group functionalized Bronsted acidic ionic liquids. J Therm Anal Calorim. 2011;103:1027–30.
Rao JC, Krishnan RV, Venkatesan KA, Nagarajan K, Srinivasan TG. Thermochemical properties of some bis(trifluoromethyl-sulfony)imide based room temperature ionic liquids. J Therm Anal Calorim. 2009;97:937–43.
Haerens K, Matthijs E, Chmielarz A, Braggen BV. The use of ionic liquids based on choline chloride for metal deposition: a green alternative? J Environ Manage. 2009;90:3245–52.
Figueiredo M, Gomes C, Costa R, Martins A, Pereira CM, Silva F. Differential capacity of a deep eutectic solvent based on choline chloride and glycerol on solid electrodes. Electrochim Acta. 2009;54:2630–4.
Li H, Wang XJ, Jia DX, Gu JS. Electrochemical preparation and characterization of Ag nanoparticles in choline chloride based ionic liquid. Chem Res. 2010;21:15–7.
Dale PJ, Samantilleke AP, Shivagan DD, Peter LM. Synthesis of cadmium and zinc semiconductor compounds from an ionic liquid containing choline chloride and urea. Thin Solid Films. 2007;515:5751–4.
Yue DY, Jing Y, Sun JH, Wang XH, Jia YZ. Structure and ion transport behavior analysis of ionic liquid analogue based on magnesium chloride. J Mol Liq. 2011;158:124–30.
Yoshimoto N, Shirai T, Morita M. A novel polymeric gel electrolyte systems containing magnesium salt with ionic liquid. Electrochim Acta. 2005;50:3866–71.
NuLi YN, Yang J, Wang P. Electrodeposition of magnesium film from BMIMBF4 ionic liquid. Appl Surf Sci. 2006;252:8086–90.
Pandey GP, Hashmi SA. Experimental investigations of an ionic-liquid-based, magnesium ion conducting, polymer gel electrolyte. J Power Sources. 2009;187:627–34.
Han J, Wu JS, Wang LS. Measurement and correlation of solubility of benzenephosphonic acid in water. Liaoning Chem Ind. 2006;6:363–5.
Abbott AP, Harris RC, Ryder KS. Application of hole theory to define ionic liquids by their transport properties. J Phys Chem B. 2007;111:4910–3.
Abbott AP, Boothby D, Capper G, Davies DL, Rasheed RK. Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids. J Am Chem Soc. 2004;126:9142–7.
Wang JY, Jiang HC, Liu YM, Hu YQ. Density and surface tension of pure 1-ethyl-3-methylimidazolium l-lactate ionic liquid and its binary mixtures with water. J Chem Thermodyn. 2011;43:800–4.
Acknowledgements
This study is financially supported with the Major Project of Chinese National Programs for Fundamental Research and Development (973 Program, No. 2010CB635100) and General Project of Natural Science Foundation of China (No. 201073217).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yue, D., Jing, Y., Ma, J. et al. Physicochemical properties of ionic liquid analogue containing magnesium chloride as temperature and composition dependence. J Therm Anal Calorim 110, 773–780 (2012). https://doi.org/10.1007/s10973-011-1960-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1960-4