Abstract
A novel flame retardant, containing silicon and nitrogen (PSiN), was synthesized, and it was used together with 9, 10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) to prepare a flame-retardant system for epoxy resins (EP). Flammability and thermal behaviors of EP systems were estimated by limited oxygen index (LOI), vertical burning test (UL-94), microscale combustion calorimetry test and thermogravimetric analysis. The results showed that synergistic effects on the flame retardancy of EP composites existed between the DOPO and PSiN. When 3 % PSiN and 7 % DOPO were incorporated, the LOI value of EP was found to be 34 %, and the UL-94 test found to be class V-0. The microstructures observed by scanning electron microscopy and FTIR indicated that the surface of the char for EP/DOPO/PSiN system holds a more cohesive and denser char structure when compared to the pure EP and EP/DOPO systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Epoxy resins (EPs) are widely used in the area of adhesives, coatings and advanced composites of the aerospace and electronic industries, because of their mechanical stiffness, toughness, superior adhesion, modulus, good chemical and corrosion resistance and excellent dimensional stability [1, 2]. EPs have a dominant position in the development of high-performance materials. However, the epoxy resins are difficult to satisfy the requirements of special engineering technology with the index (LOI) of 21 %, which limits the application of epoxy resin in many important areas, so the flame retardancy of EP needs to be improved [3–5].
Although halogen flame retardants have good flame retardancy on epoxy resins, the flame retardants would produce a large amount of toxic and corrosive smoke in combustion process [6]. So, low-smoke and halogen-free flame retardants of epoxy resin have attracted increasing attention [7–9].
Researches have shown that the addition of relatively small amounts of silicon compounds to various polymeric materials can improve their flame retardancy [10, 11]. And introducing silicon element and its groups into epoxy can also improve some other properties of the epoxy resins, such as thermal stability, high resistance to thermal oxidation, low surface energy and low toxicity [12, 13].
Phosphorus-containing compounds or resins have been demonstrated as effective flame retardants for epoxy resins, attributed to their advantages including high flame retardant efficiency and low production of corrosive and toxic gases in flames [14]. Among them, 9, 10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) has attracted intensive attention due to its high flame retardant efficiency and thermal properties. Hence, various DOPO-based epoxy resins, curing agents and additives have been reported [15–22].
Moreover, effective synergistic effect of silicon and phosphorus on enhancing char formation and improving flame retardancy of the polymers was also observed [23]. A novel approach to obtain epoxy resins with excellent thermal properties as well as high flame retardancy was reached when simultaneously incorporating silicon and phosphorus into the epoxy resins.
In this work, a novel organic silicon flame retardant containing silicon and nitrogen (PSiN) was synthesized and incorporated into the epoxy resins simultaneously with DOPO. The PSiN can use as a curing agent for epoxy resin. The synergistic effect of these components on the flame retardancy and thermal properties of epoxy resins was studied. The compatibilization, thermal degradation behavior and the flame retardant effect were investigated by scanning electron microscopy (SEM), microscale combustion calorimetry (MCC), differential scanning calorimetry (DSC), thermogravimetric analysis (TG) and limiting oxygen index (LOI).
Experimental
Materials
Diglycidyl ether of bisphenol A (DGEBA, EP, epoxy value = 0.44 mol/100 g) was supplied by Wuxi Bluestar Chemical Co., Ltd., China. 9, 10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) was supplied by Huizhou Shengshida Chemical Co., Ltd (China). N-(β-aminoethyl)-γ-aminopropylmethyl dimethoxysilane (KH-602) and dimethoxydimethylsilane were acquired from Nanjing Union Silicon Chemical Co., Ltd (Nanjing, China) and Bluestar Chemical Research Institute (Chengdu, China), respectively.
Synthesis of PSiN
A 250-mL three-necked round-bottom flask equipped with a stirrer, a thermometer and a condenser was charged with 12 g (0.1 mol) dimethoxydimethylsilane, 20.6 g KH-602 (0.1 mol) and 5 g H2O. Then, a suitable amount of ammonia was added into the flask as catalyst. After being maintained at room temperature for 20 min, the solution was heated to 60 °C and kept refluxed until there is no increase in viscosity for the reaction system. After being cooled to room temperature, the raw product was washed by ethanol and n-hexane to remove the residual KH-602. The product was dried in a vacuum at 110 °C. The synthesis route is illustrated in Fig. 1.
Blending of epoxy resin
EP/PSiN/DOPO was prepared as the following steps. First, appropriate amounts of epoxy were with DOPO stirring at 120 °C until the DOPO was in molten state. Second, stoichiometric DDM was casted into the blend above to dissolve at 90 °C. Third, the appropriate amount of PSiN and DDM was put into the epoxy at 60 °C. Compositions of the EP are shown in Table 1. Subsequently, the blend was put into a mold for curing and postcuring at oven as the following procedure: 80 °C/2 h + 120 °C/2 h + 150 °C/2 h. The blend molded into standard testing bars for further test.
Instrumental analyses
FTIR spectroscopy was applied to a Nicolet IS10 Fourier transform infrared (FTIR) spectrometer using KBr pellets. 1H NMR (400 MHz) spectrum were recorded on a FT-80A NMR using CDCl3 as the solvent. The Q-200 (TA Instrument) was used for differential scanning calorimetry (DSC) under nitrogen atmosphere using a scanning rate of 10 °C min−1. The morphologies of the fractured surfaces of samples and the surface morphology of the char obtained after the LOI test were observed by using SEM. Thermogravimetric analysis (TG) was performed on a Mettler Toledo instrument TG/DSC1 at a heating rate of 10 °C min−1. About 10 mg of the sample was examined under pure nitrogen or air condition at a flowing rate of 60 mL min−1 at temperatures ranging from 30 to 800 °C. LOI values of all samples were obtained at room temperature on an oxygen index instrument (XYC-75) produced by Chende Jinjian Analysis Instrument Factory, according to GB/T 2406-93 standard. The dimensions of all samples were 130 × 6.5 × 3 mm3. LOI value was an important parameter for evaluating the ease of extinguishment of polymeric materials in the same condition. It denotes the lowest volume concentration of oxygen sustaining candle burning of materials in the mixing gases of nitrogen and oxygen. A Govmark MCC-2 microscale combustion calorimetry (MCC) was used to determine the flammability characteristics of EP systems according to ASTM D7309-07. Samples of 4–6 mg were heated to 700 °C at a heating rate of 1 °C s−1 in a stream of nitrogen flowing at 80 cm3 min−1. The volatile and anaerobic thermal degradation products in the nitrogen gas stream were mixed with a 20-cm3 min−1 stream of pure oxygen before entering a 900 °C combustion furnace.
Results and discussion
Characterization of PSiN
Figure 2 presents the FTIR spectrum of the synthesized PSiN. The absorption bands at 2960 cm−1 correspond to vibrations of C–H. The peaks at 1022 and 1087 cm−1 are associated with the vibrations of Si–O–Si. The absorptions at 1260 and 800 cm−1 are assigned to Si–CH3. The absorptions at 1476 cm−1 are assigned to –CH3 and –CH2. The peaks at 3356 cm−1 are associated with the vibrations of –NH2. The information above confirms that the target product was synthesized successfully.
So as to further determine the structure of PSiN. Figure 3 shows the 1H NMR spectrum of PSiN (ppm): 0.04 ppm (CH3–Si–CH3), 0.5 ppm (Si–CH2−), 1.3–1.6 ppm (–Si–CH2–CH2− N–), 1.8–2.1 ppm (–NH), 2.5–3.0 ppm (N–CH2) and 7.2 ppm (CDCl3). All these characteristic 1H NMR bands indeed match the PSiN structure. These suggest that PSiN is successfully processed. On the other hand, the molar ratio (X:Y) of dimethyldimethoxysilane to KH602 was determined by the ratio of the area of peak a (–CH3 in dimethyldimethoxysilane) to that of peak f (–NH in KH602), and the result indicates the value of X:Y is 1.
Differential scanning calorimetry (DSC)
Figure 4 gives DSC curves of cured EP systems. All of the epoxy systems exhibit single glass transition temperature (T g). It is well known that an absolutely miscible blend presents only one T g peak. This single T g phenomenon indicates a homogeneous morphology of these hybrid epoxy systems. This homogeneous morphology provides possibility to synergistic effect of DOPO and PSiN on the flame retardancy of epoxy resins.
Flammability
The flame retardancy of cured EP, EP/DOPO and EP/PSiN/DOPO was evaluated by measuring their LOI values. Data are listed in Table 1. It is clear that a significant increase in LOI value (from 21.5 to 32 %) was observed when DOPO was utilized in the epoxy curing compositions. This indicates that incorporating phosphorus has an efficient effect on leveling up the flame retardancy of the epoxy resins. From the UL-94 results, it can be observed that when the total amount of DOPO was 7 %, the UL-94 of the system was only V-1 rating. While the total amount of DOPO was 10 %, the UL-94 of the system was V-0 rating, but the LOI value decreased. From Table 1, when the PSiN contents of the EP remain constant and DOPO contents of the EP are added from 4 to 7 %, the LOI values of the EP system reached a maximum of 34 %, and class V-0 of UL 94 was passed. PSiN will produce less smoke in the combusting process. Meanwhile, the mixtures with PSiN can keep the original shape and form hollow shell after burning.
Microscale combustion calorimetry test
The heat release rate (HRR), the peak heat release rate (PHRR), heat release capacity (HRC) and the total heat release (THR) are important parameters in quantitative material flammability analysis. The dynamic flammability data detected by microscale combustion calorimeter of EP and flame retardant EP samples are shown in Fig. 5 and Table 2. Figure 5 contains curves of the heat release rate (HRR) of EP systems. A sharp peak appeared in the HRR of EP with a peak heat release rate (PHRR) of 467.5 W g−1, while EP/PSiN and EP/DOPO systems showed a slower burning rate with the peak reduced to 415, 326 W g−1, respectively. EP/PSiN/DOPO showed the slowest burning rate with the PHRR of 283 W g−1. The results suggest that the addition of PSiN and DOPO has a considerable effect on the stability of EP. The heat release capacity of EP, EP/PSiN and EP/DOPO systems are presented in Table 2. The heat release capacity (HRC) of EP/PSiN and EP/DOPO was lower than pure EP. The heat release capacity of EP/PSiN/DOPO was lowest among the EP systems. This lower HRC value indicates that a part of the EP system was not completely combusted, probably undergoing a carbonization process. It is believed that some char residues formed in the thermal decomposition process of EP, which could lead the heat release rates, decrease. This effectively improves the flame retardancy of the matrix.
Thermal analysis
The thermal behaviors of the composites are investigated by TG under both air and nitrogen atmospheres. The typical data are obtained from TG and DTG curves as shown in Table 3. T 5% represents the temperature at which mass loss is 5 % for the system (the initial decomposition temperature), T max1 represents the temperature of the first maximum mass loss rate for the system, T max2 represents the temperature of the second maximum mass loss rate for the system, R max represents the maximum mass loss rate for the system and Y c represents the char yields at 800 °C [24].
Figures 6 and 7 show the TG and DTG curves of cured EP (Sample 1), EP/PSiN (Sample 2), EP/DOPO (Sample 4) and EP/PSiN/DOPO (Sample 7) resins in air atmosphere, and the data from the curves are shown in Table 3. TG curves showed two decomposition stages (Fig. 6), in which the first stage was assumed as the thermal degradation of the polymer network and the second one as the oxidation process of the char moiety. Epoxy resins have a very small amount of volatiles when the temperature was increased to 370 °C. As the temperature increases, mass loss increases remarkably and a large quantity of volatiles is produced until almost no residue is left at 800 °C. The T 5% of the EP/PSiN/DOPO and EP/DOPO is lower than epoxy resins. This can be explained as follows: The initial decomposition of DOPO occurs at inferior temperature. The low bond energy of P–O and P–C leads to the low initial decomposition temperature. However, with the addition of PSiN, the T 5% increased which might be due to the fact that decomposition of DOPO at inferior temperature leads to the formation of the silicone-containing group, which will participate in the cross-linked carbonization. And PSiN can convert the usual organic decomposition to partially inorganic decomposition by forming the carbon–silicon residue to act as thermal insulation and to prevent gas evolution and achieve ultimate improvement on flame retardation of this phosphorous–silicone-containing epoxy system [25–27]. In the temperature range of 600–800 °C, the EP/PSiN/DOPO (Sample 7) shows the highest char residues than the sum of EP/PSiN and EP/DOPO, indicating the synergistic effect between phosphorus and silicone. From Fig. 6, the DTG curves show that maximum mass loss rate was lower with adding of PSiN. This fact demonstrated that PSiN and DOPO could decrease the decomposition rate of EP obviously. Those phenomena may contribute great to high flame retardancy such as LOI and UL-94 results.
Figures 8 and 9 show the TG and DTG curves of cured EP (Sample 1), EP/PSiN (Sample 2), EP/DOPO (Sample 4) and EP/PSiN/DOPO (Sample 7) resins in nitrogen, respectively. It can be seen that the degradation processes of all the samples are very different from those under air atmosphere. From the DTG curves, it can be found that the mass loss rate of the flame retardant systems is lower than that of pure epoxy resins. Moreover, the char yield increases from 18 to 23 % at 800 °C. Those indicate that the incorporation of PSiN and DOPO can enhance the char yield, which can protect the underlying materials from further decomposition.
Generally, the PSiN and DOPO could improve the char residues of epoxy resins in both air and nitrogen atmospheres and the char layers are the critical factors which could slow down heat and mass transfer between the gases and condensed phases. It is reported that the silicon usually immigrates to the surface of the char and plays the role of char enhancer [28]. Due to the synergistic effect between phosphorus and silicon, this exhibits high flame retardancy and this can be suggested by the LOI and UL-94 results.
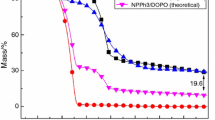
Figure 10 shows the experimental and theoretical TG curves of Sample 7 system in air. The theoretical curve was calculated based upon the mass percentage of the ingredients in the EP system. As could be seen, when the temperature was below 450 °C, the experimental and theoretical curves were almost same. However, the experimental residual char became more than the theoretical one after 450 °C. It could be deduced some high-temperature stable material was produced in the char. This fact was the proof of the synergistic effect of DOPO and PSiN and this synergistic effect could improve the char formation ability of EP.
It is well known that the effective protection of the char layer could improve the flame retardancy; thus, it is necessary to evaluate the char layers so as to further investigate the mechanism [29]. To further investigate the effect of DOPO and PSiN on the char formation of EP system during combustion, the morphologies of the char residues were examined by SEM. The SEM micrographs of the char residue of EP, EP/PSiN and EP/PSiN/DOPO are shown in Fig. 11. As for the char of pure EP, it displayed uneven and incompact surface char residues. This poor char cannot efficiently act as a barrier to shield the underlying polymer from heat and air. However, the char of EP with both DOPO and PSiN, illustrated in Fig. 11, was compact, smooth and dense. This structure of the char for EP composites could prevent heat transfer between the flame and the substrate and thus protect the underlying materials from further burning and pyrolysis, so they had much higher LOI values. In addition, EP/PSiN showed a smoother char than EP, and EP/DOPO showed a more connective char than EP. This indicates that phosphorus provides a tendency for better char formation; silicon provides an enhancement of the thermal stability of the char. It can be concluded that a significant synergistic effect existed between DOPO and PSiN when they were applied. The synergistic effects of DOPO and PSiN result in the condensed and intumescent char layers, which are in accordance with the TG results in air atmosphere.
Structural analysis of the combustion residue by FTIR
To further clarify the structure of the flame-resistant barrier on the surface, the EP (Sample 1), EP/PSiN (Sample 2) and EP/DOPO/PSiN (Sample 7) systems were analyzed after combustion using infrared (IR) spectroscopy. The IR spectra of this system are shown in Fig. 12. The broad peak at around 1026 cm−1 appeared which are due to the stretching vibration of P–O, the Si–O–Si or benzene ring bonds [13]. Meanwhile, sharp absorption peaks appearing at 1408 cm−1 is assigned to the stretching vibrations of C=C in the aromatic compounds. Moreover, the broad vibration bands at 889 cm−1 are due to the superposition of stretching vibrations of P–O–P or P–O–Ph bonds. And 3450 cm−1 becomes broader, which was assigned to the stretching mode of –OH from the P–OH group, indicating the presence of alcohol groups in the char. The P–O–P groups can be regarded as the cross-links which link to different aromatic species and reinforce carbon layers [30, 31]. Based on the observed results, it is proposed that DOPO and PSiN would form some silica–phosphate char residues with the matrix, which largely consists of condensed aromatic compounds and Si–O units [2]. The char acts as a good insulating barrier, which could well protect the EP matrix.
Conclusions
This paper presents the preparation of novel polysiloxane (PSiN) flame retardants containing nitrogen and silicon. The PSiN was used together with DOPO to prepare a flame-retardant system for epoxy resins, and the flame retardancy of EP composites was characterized. The results showed that synergistic effects on the flame retardancy of EP composites existed between the DOPO and PSiN. The char residues of EP/PSiN/DOPO composites were increased, and the highest char residues were obtained in air atmosphere when the DOPO was 7 mass% and PSiN was 3 mass%, which was in accordance with the highest LOI and UL-94 results. The SEM and FTIR results of pyrolysis products indicated that resulting in significant improvements in the flame retardancy of epoxy resins.
References
Liu Q, Bao X, Deng SQ. The investigation of methyl phenyl silicone resin/epoxy resin using epoxy-polysiloxane as compatibilizer. J Therm Anal Calorim. 2014;118:247–54.
Bao X, Cai XF. Synergistic effect of methyl phenyl silicone resin and DOPO on the flame retardancy of epoxy resins. J Therm Anal Calorim. 2014;118:369–75.
Ahmada S, Gupta AP, Sharmin E, Alam M, Pandey SK. Synthesis, characterization and development of high performance siloxane-modified epoxy paints. Prog Org Coat. 2005;54:248–55.
Wu CS, Liu YL, Chu YS. Epoxy resins possessing flame retardant elements from silicon incorporated epoxy compounds cured with phosphorus or nitrogen containing curing agents. Polymer. 2002;43:4277–84.
Jiang J, Chen YB, Liu Y, Wang Q. Intergrowth charring for flame-retardant glass fabric-reinforced epoxy resin composites. J Mater Chem A. 2015;3:4284–90.
Yuan DD, Yin HQ, Cai XF. Synergistic effects between silicon-containing flame retardant and potassium-4-(phenylsulfonyl)benzene-sulfonate (KSS) on flame retardancy and thermal degradation of PC. J Therm Anal Calorim. 2013;114:19–25.
Chen XL, Jiao CM. Synergistic effects of hydroxy silicone oil on intumescent flame retardant polypropylene system. Fire Saf J. 2009;44:1010–4.
Lu SY, Hamerton I. Recent developments in the chemistry of flame retardant composites matrices. Prog Polym Sci. 2002;27:1661–712.
Shen H, Wang YH, Mai KC. Non-isothermal crystallization behavior of PP/Mg(OH)2 composites modified by different compatibilizers. Thermochim Acta. 2007;457:27–34.
Wang WJ, Perng LH, Hsiue GH, Chang FC. Characterization and properties of new silicone-containing epoxy resin. Polymer. 2000;41:6113–22.
Liu SM, Lang XM, Ye H. Preparation and characterization of copolymerized aminopropyl/phenylsilsesquioxane microparticles. Eur Polym J. 2005;41:996–1001.
Chiang CL, Ma CCM. Synthesis, characterization, thermal properties and flame retardance of novel phenolic resin/silica nanocomposites. Polym Degrad Stab. 2004;83:207–14.
Qian XD, Song L, Yuan BH, Yu B. Organic/inorganic flame retardants containing phosphorus, nitrogen and silicon: preparation and their performance on the flame retardancy of epoxy resins as a novel intumescent flame retardant system. Mater Chem Phys. 2014;143:1243–52.
Hsiue GH, Liu YL, Tsiao J. Phosphorus-containing epoxy resins for flame retardancy V: synergistic effect of phosphorus–silicon on flame retardancy. J Appl Polym Sci. 2000;78:1–7.
Lin CH, Feng CC, Hwang TY. Preparation, thermal properties, morphology, and microstructure of phosphorus containing epoxy/SiO2 and polyimide/SiO2 nanocomposites. Eur Polym. 2007;43:725–42.
Wang XD, Zhang Q. Synthesis, characterization, and cure properties of phosphorus-containing epoxy resins for flame retardance. Eur Polym. 2004;40:385–95.
Lin CH, Wu CY, Wang CS. Synthesis and properties of phosphorus-containing advanced epoxy resins. II. J Appl Polym Sci. 2000;78:228–35.
Wang CS, Shieh JY. Synthesis and properties of epoxy resins containing 2-(6-oxid-6H-diben <c,e> <1,2> oxa-phosphorin-6-yl)1,4-benzenediol. Polymer. 1998;39:5819–26.
Cho CS, Fu SC, Chen LW, Wu TR. Aryl phosphinate anhydride curing for flame retardant epoxy networks. Polym Int. 1998;47:203–9.
Liu YL, Wu CS, Hsu KY, Chang TC. Flame-retardant epoxy resins from o-cresol novolac epoxy cured with a phosphorus containing aralkyl novolac. J Polym Sci Polym Chem. 2002;40:2329–39.
Wang CS, Lin CH. Properties and curing kinetic of diglycidyl ether of bisphenol A cured with a phosphorus-containing diamine. J Appl Polym Sci. 1999;74:1635–45.
Levchik SV, Weil ED. Thermal decomposition, combustion and flame retardancy of epoxy resins—a review of the recent literature. Polym Int. 2004;53:1901–29.
Hsiue GH, Liu YL, Liao HH. Flame retardant epoxy resins: an approach from organic-inorganic hybrid nanocomposites. J Polym Sci A Polym Chem. 2001;39:986.
Gu AJ, Liang GZ. Thermal degradation behaviour and kinetic analysis of Epoxy/montmorillonite nanocomposites. Polym Degrad Stab. 2003;80:383–91.
Kanai H, Sullivan V, Auerback A. Impact modification of engineering thermoplastics. J Appl Polym Sci. 1994;53:527–41.
Kambour RP, Ligon WV, Russell RP. Enhancement of the limiting oxygen index of an aromatic polycarbonate by the incorporation of silicone blocks. J Polym Sci C Polym Lett. 1978;16:327–33.
Durga G, Narula AK. Curing and thermal behaviour of diamide–diimide–diamines based on l-phenylalanine with epoxy blends containing phosphorus/silicon. J Therm Anal Calorim. 2012;109:345–53.
Chen YJ, Zhan J, Zhang P, Nie SB, Lu HD, Song L, Hu Y. Preparation of intumescent flame retardant poly(butylene succinate) using fumed silica as synergistic agent. Ind Eng Chem Res. 2010;49:8200–8.
Morgan AB, Harris RH, Kashiwagi T, et al. Flammability of polystyrene layered silicate (clay) nanocomposites: carbonaceous char formation. Fire Mater. 2002;26:247–53.
Bugajny M, Bourbigot S, Le Bras M, et al. The origin and nature of flame retardance in ethylene vinyl acetate copolymers containing hostaflam AP 750. Polym Int. 1999;48:264–70.
Wang ZZ, Lv P, Hu Y, Hu KH. Thermal degradation study of intumescent flame retardants by TG and FTIR: melamine phosphate and its mixture with pentaerythritol. J Anal Appl Pyrol. 2009;86:207–14.
Acknowledgements
We would like to thank the generous financial support by the following grant: Applied basic research project of Sichaun Technology Department, Grant No. 2014JY0138.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, L., Wang, Y., Liu, Q. et al. Synergistic effects between silicon-containing flame retardant and DOPO on flame retardancy of epoxy resins. J Therm Anal Calorim 123, 1343–1350 (2016). https://doi.org/10.1007/s10973-015-5046-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-5046-6