Abstract
A novel flame-retarded epoxy resins system is prepared by copolymerizing diglycidyl ether of bisphenol A (EP) with tris(3-nitrophenyl) phosphine (NPPh3), 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO), and 4,4-diaminodiphenylmethane (DDM). The thermogravimetric curves suggest that there is an obvious synergistic effect between NPPh3 and DOPO. Flame-retardant properties of the cured products are evaluated using limited oxygen index (LOI) and vertical burning tests (UL-94). The results indicate that the flame retardancy of NPPh3/DOPO/EP thermosets is enhanced. 2%NPPh3/4%DOPO/EP achieves a LOI value of 33.8% and V-0 rating in UL-94 test. The thermal stability of the EP composites is detected by thermogravimetric analysis (TG) and differential scanning calorimetry. The results demonstrate that the thermal stability of NPPh3/DOPO cured epoxy resins displays an improvement in the high-temperature region and the glass transition temperature decreases slightly compared with pure EP. The pyrolytic gases are characterized using thermogravimetric analysis/infrared spectrometry (TG-FTIR) in an air atmosphere. The gaseous species produced by the flame-retarded EP composites are the same as those from EP. Additionally, the morphology and the structure of char residues are studied by scanning electron microscopy and Fourier transform infrared spectra (FTIR). The morphology of the residual char for flame-retarded EP composites shows a compact, smooth, and tight structure. These outstanding integrated properties will make EP composites attractive for practical applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Epoxy resins (EP) are a well-received thermoset, which possesses advantages of excellent mechanical properties, remarkable low shrinkage on cure, outstanding electrical, chemical resistance properties, and low manufacturing cost [1,2,3]. Therefore, they are widely used in automotive, defense, aerospace, and electrical industry [4, 5]. However, pure EP material has a low LOI value and is flammable [6]. In order to extend the applications of EP, it is important to develop effective flame-retardant systems.
Over the past decades, halogenated flame retardants have been widely used in epoxy resins, but they pollute the environment [7]. Therefore, the focus on the flame retardants is shifted to halogen-free flame retardants [8]. 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) is an important phosphorus flame retardant, which has high reactivity and good thermal stability [9]. The use of DOPO on epoxy resins to prepare flame-retardant thermoset has been reported [10, 11]. When DOPO works with other flame retardants, they can further enhance the flame retardancy of EP [12]. Studies about DOPO have focused on the additive constructed by several functional groups such as silicon [13], triazene [14], and maleimide [15]. These additives confirm synergistic flame-retardant effect with DOPO, thus they endow matrix with high flame retardancy. Additives containing nitro group can exert flame-retardant effect [16], however, if a combination of nitro compound with DOPO will result in enhanced flame retardancy is unknown.
In this paper, tris(3-nitrophenyl) phosphine (NPPh3), a nitro group containing flame retardant, is synthesized. A novel flame-retarded epoxy resins system is prepared by copolymerizing EP with NPPh3, DOPO, and DDM. Synthesis effects between NPPh3 and DOPO on the flame retardancy and thermal properties of EP are studied. And the flame-retardant mechanism is discussed in detail.
Experimental
Materials
Diglycidyl ether of bisphenol A (DGEBA, EP, epoxy value = 0.44) was supplied by Wuxi Bluestar Chemical Co. Ltd. China. Triphenylphosphine (PPh3, 9, 10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide (DOPO) (Scheme 1), 4,4-diaminodiphenylmethane (DDM), sodium bicarbonate, sulfuric acid, and nitric acid were purchased from Chengdu Kelong Chemical Reagent Factory. Ethanol was obtained from Chengdu Changlian chemical reagent Co. Ltd. China.
Synthesis of tris(3-nitrophenyl) phosphine (NPPh3)
2.62 g (0.01 mol) PPh3 and 20 g (0.2 mol) sulfuric acid were added to a 100-mL three-necked round bottom flask equipped with a thermometer, a stirrer, and a constant pressure dropping funnel. PPh3 was dissolved in sulfuric acid with stirring at room temperature. Then, the temperature of the mixed system was reduced to below 10 °C with an ice-salt bath and the mixed solution of sulfuric acid (30 g, 0.3 mol) and nitric acid (6 g, 0.06 mol) was slowly added dropwise over 30 min. After that, the temperature was raised to room temperature and kept for 6 h. Finally, the product was precipitated by pouring the reaction mixture over an excess of ice-cold water, neutralizing with a saturated solution of sodium bicarbonate, washing with water and filtering. The reaction formula is shown in Scheme 2. Yield: 3.9 g (89%).
Preparation of epoxy resins
NPPh3/DOPO/EP was prepared as the following steps. NPPh3 was mixed with ethanol into a paste at first. Then, the mixture was put into EP and dried to eliminate ethanol in rotary evaporators. Subsequently, DOPO was fed into the blend at 150 °C until it was a molten state, and DDM was added relative to the amount of EP at the same temperature. Compositions of the EP are shown in Table 1. After that, the blend was poured into the prepared mold for curing and postcuring at oven as the following procedure: 80 °C/2 h + 120 °C/2 h + 150 °C/1 h.
Characterization
The limiting oxygen index (LOI) data of all samples were obtained on an oxygen index instrument (XYC-75) produced by Chengde Jinjian Analysis Instrument Factory according to ASTMD2863-08 standard with a specimen dimension of 130 × 6.5 × 3.2 mm3 at room temperature. Vertical burning tests (UL-94) were carried out by a CZF-2 instrument (Jiangning Analysis Instrument Company, China) with sample dimension of 125 × 12.5 × 3 mm3 according to UL-94 ASTMD635-77 standard at room temperature.
Thermogravimetric analysis (TG) was performed on a Netzsch TG209 F1 at a heating rate of 10 °C min−1. 5–10 mg of the sample was examined under air and nitrogen atmosphere at a flowing rate of 60 mL min−1 of temperature from 30 to 800 °C.
The Q-200 (TA Instrument) was used for differential scanning calorimetry (DSC) under nitrogen atmosphere using a scanning rate of 10 °C min−1.
Thermogravimetric analysis/infrared spectrometry (TG-FTIR) was carried out on a Mettler Toledo TG/DSC 1 STARe System thermogravimeter couple with a Nicolet IS10 FTIR spectrophotometer at a linear heating rate of 20 °C min−1 under air within 50–800 °C.
Scanning electron microscopy (SEM) analyses were used to study the morphologies of residues obtained from combustion by UL-94 using a FEI QuanTa-250 SEM.
Fourier transform infrared spectra (FTIR) were obtained on a Nicolet Magna-FTIR 560 spectrometer (Nicolet Instrument Co, USA) with KBr pellets.
Tensile tests were carried out on an Instron M5967 (Intron, USA) testing machine. Samples were tested with a crosshead speed of 20 mm min−1.
Results and discussion
Synergistic effect
In order to investigate the synergistic effect between NPPh3 and DOPO, the thermal degradation behaviors of NPPh3/DOPO are studied by thermogravimetric analysis. Figure 1 shows the TG curves of NPPh3, DOPO, and NPPh3/DOPO (1:2) systems. The theoretical curve is calculated based upon the mass percentage of the ingredient. The formula is as follows:
(wt% refers to the corresponding proportion of ingredients; Y cal is the theoretical value of carbon residue; Y exp is the actual amount of carbon residue.)
As shown in Fig. 1, NPPh3 is thermal stable below 290 °C. The thermal degradation of NPPh3 occurs in a single step with the main peak of mass loss at 378.7 °C, and its char residual at 800 °C is 28.16%. The degradation process of DOPO also exhibits one step from 174 to 260 °C with a maximum degradation rate at 245.4 °C. However, it decomposes completely at 800 °C, which means that DOPO shows poor charring ability. When NPPh3 is combined with DOPO, the TG curves between experimental and theoretical exhibit marked difference. Compared with the theoretical curve which undergoes a rapid mass loss in the range of 160–250 °C, actual mass loss of the experimental curve is much lower. Furthermore, the experimental mass of the residual char surpasses the theoretical one after 160 °C and has an improvement of 19.6% at 800 °C. It can be speculated that a chemical interaction between NPPh3 and DOPO exists at lower temperature and promotes char formation.
Flame retardancy
The flame-retardant properties of EP thermosets are detected by UL-94 and LOI tests; the related data are listed in Table 2. It can be seen that the LOI values of 6%NPPh3/EP and 6%DOPO/EP samples increase to 26.5 and 31.2%, respectively, and that of the pure EP is only 23%. When NPPh3 and DOPO are incorporated into EP, LOI value of NPPh3/DOPO/EP systems is changed with the ratio of NPPh3 to TPP. These values increase with an increasing ratio of NPPh3 to TPP, then decrease with a further increase in the ratio. When the mass ratio of NPPh3: DOPO is 1:2, the LOI value of the NPPh3/DOPO/EP system reaches a maximum of 33.8%, and class V-0 of UL-94 is passed. These results also prove that there is a synergistic effect of NPPh3 and DOPO when they are applied in epoxy resins, and the effect has a good influence on flame retardancy.
Thermal analysis
Figure 2 shows the TG and DTG curves of EP, 6%NPPh3/EP, 6%DOPO/EP, and 2%NPPh3/4%DOPO/EP under N2 atmosphere. The corresponding T 5%, R peak, T max, and Y c are summarized in Table 3. As can be seen, NPPh3 and DOPO cause the decomposition of EP in advance, which can be attributed to the low bond energy of P–C [17]. And T 5% of 6%NPPh3/EP is lower than 6%DOPO/EP probably because NPPh3 contains more P–C in it. Meanwhile, the R 1peak of 6%NPPh3/EP and 6%DOPO/EP is decreased comparing with pure EP in Table 3 due to the previously formed char layer which protects the underlying epoxy resins matrix from further degradation [18]. With the addition of DOPO, char residue at 800 °C does not seem to increase significantly comparing with pure EP. However, NPPh3 promotes the formation of more residue than DOPO does. When NPPh3 and DOPO are incorporated commonly into EP at a certain ratio, flame-retardant epoxy resins show only one mass loss stage as pure EP. The initial decomposition temperature is shifted to a lower temperature compared with EP, 6%NPPh3/EP, and 6%DOPO/EP. This can be attributed to the earlier decomposition of NPPh3 and DOPO and the synergistic effects between them. Furthermore, T 1max of 2%NPPh3/4%DOPO/EP is decreased in a broad region with loss mass rate of 9.56% min−1 and its char residue at 800 °C is 17.87%.
The pyrolysis process under air atmosphere is similar to combustion condition [19]. The TG and DTG curves of EP, 6%NPPh3/EP, 6%DOPO/EP, and 2%NPPh3/4%DOPO/EP in air atmosphere are shown in Fig. 3, and the detailed data are summarized in Table 4. As can be seen, the pure EP starts to decompose at 352.5 °C and its residual char at 800 °C is 0%. Compared with only one decomposition stage that takes place in nitrogen atmosphere, the thermal degradation of EP in air can be divided into two stages. The first thermal decomposition occurs between 350 and 460 °C, corresponding to the thermal degradation and carbonization of the polymer network [20]. The second stage is from 460 to 640 °C, which is attributed to further oxidation of the formed residual char with poor thermal stability [21]. With the incorporation of NPPh3, the value of T 5% is 291.5 °C, and its thermal degradation occurs in three steps. The first stage is assumed as the thermal degradation of the NPPh3; the second stage is mainly the degradation of EP; and the third stage is due to the char formed in the second stage which behave unstable stability [22]. For 6%DOPO/EP, the value of T 5% is 336.4 °C. The initial thermal decomposition occurs between 300 and 460 °C; the second stage is from 460 to 640 °C. And its main pyrolysis peaks exhibit at 362.6 and 561.4 °C, respectively. In addition, the value of char residues of 6%NPPh3/EP at 800 °C is same as that of 6%DOPO/EP which Y c is zero. When NPPh3 and DOPO are incorporated into EP, 2%NPPh3/4%DOPO/EP exhibits two-stage decomposition processes. It is necessary to point out that 2%NPPh3/4%DOPO/EP shows good thermal stability at high temperature and its char residue at 800 °C is 4.62%, which is higher than that of 6%NPPh3/EP and 6%DOPO/EP. The result indicates that NPPh3 and DOPO play an important role in the condensed phase of epoxy resins, which lead to efficient enhancement of the mass of char residue.
The glass transition temperatures (T g) of the blending systems are measured by DSC. The DSC curves of the samples are displayed in Fig. 4. As can be seen, the samples all exhibit a single T g. When NPPh3 is added to EP, 6%NPPh3/EP shows a lower T g than that of pure EP. These results can be partially explained by two possible factors, which are the addition of NPPh3 reduces the cross-linking density of EP and the cohesive forces between the EP molecules are loosened by the NPPh3 particles. With the addition of DOPO, 6%DOPO/EP exhibits much lower T g. It is supposed that the cross-linking density of EP decreases with DOPO, which is introduced by means of end-capping reaction [23]. Test shows that T g value of NPPh3/DOPO/EP is lower than 6%NPPh3/EP and 6%DOPO/EP. These results are due to the addition reaction of NPPh3 and DOPO.
Gas phase analysis
The TG-FTIR is used to analyze the gas products formed during the thermal degradation [24]. In order to further explore the thermal degradation mechanism, the pyrolytic gas species produced by EP and 2%NPPh3/4%DOPO/EP are detected in an air atmosphere. The FTIR spectra of pyrolytic gas products at the maximum decomposition rates and 3D TG-FTIR spectra of the pyrolysis products are shown in Figs. 5 and 6. As shown in Fig. 5, 2%NPPh3/4%DOPO/EP releases the similar decomposition products with that of EP. The major pyrolytic gases are phenol derivatives/water (3735 cm−1) [25], CO2(2359 cm−1) [2, 26], and aromatics (669 cm−1) [27]. No other characteristic absorptions of the gas products for NPPh3/DOPO, it suggests that most of the decomposition products of NPPh3/DOPO remain in the condensed phase. Releases of CO2 and aromatics as a function of temperature are shown in Fig. 7. The release of CO2 and aromatics of EP is clearly divided into two steps, whereas that of 2%NPPh3/4%DOPO/EP is in one step. The signal intensity of CO2 and aromatics first increase with a rise of temperature. Then, the signal intensity decline gradually after the maximum degradation rate [28]. In addition, the absorbance intensity of CO2 and aromatics of 2%NPPh3/4%DOPO/EP are lower than that of EP, which means the increase in char residual. The fact is consistent with the results of the thermogravimetric analysis discussed above.
Morphology and chemical analyses of residual char
In order to explore how the structure of char determines the flame retardancy of EP, we investigate the residues of char after UL-94 by SEM. Figure 8 presents SEM micrographs of char residues of EP and 2%NPPh3/4%DOPO/EP. It is not difficult to discern the differences of the chars between them. The char of EP presents a continual char layer which has many protrusions on it. However, the char surface of EP with both NPPh3 and DOPO, as shown in Fig. 8b is compact, smooth, and tight. This structure could serve as a barrier against heat and oxygen diffusion to the surface of burning item, which endows the materials with much higher LOI values.
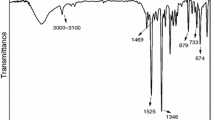
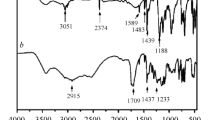
Figure 9 shows the FTIR spectra of the char residues for EP and 2%NPPh3/4%DOPO/EP after UL94 test. The absorbance peaks at 3434, 2800–3000, 1627, and 1508 cm−1 appear simultaneously in both spectrograms. The absorption peak at 3434 cm−1 assigning to N–H and O–H, which is attributed to the ammonium compounds and the pyrolysis products of the hydroxyl compounds [29]. The absorbance peaks appearing at 2800–3000 and 1627 cm−1 imply the formation of aromatic structure [20]. It must be noted that for the peak of 1508 cm−1, it assigns to the aromatic ring in the spectra of EP. However, in 2%NPPh3/4%DOPO/EP, the absorption peak at 1508 cm−1 is belonging to N–O stretching peak of nitro group [30]. Besides, new peaks at 1362, 1236, 1179, 1104, and 1039 cm−1 appear in the FTIR spectrum of 2%NPPh3/4%DOPO/EP. The absorbance peak at 1362 cm−1 can be assigned to N–O band. Peak at 1236 cm−1 indicates the existence of P=O [31]. And the peaks at 1179, 1104, and 1039 cm−1 are due to the P–O bond [32]. The discussion above further discloses the flame retardancy of DOPO and NPPh3 in the condensed phase.
Mechanical properties
Tensile strength is a measurement of the force required to pull a sample to the point where it breaks and reflects the fracture resistance of materials. As shown in Fig. 10, EP shows a tensile strength of 51.60 MPa. The incorporation of NPPh3 decreases the tensile strength of EP. However, the tensile strengths of 6%DOPO/EP and 2%NPPh3/4%DOPO/EP decrease more rapidly than that of 6%NPPh3/EP. The tensile strengths of 6%DOPO/EP and 2%NPPh3/4%DOPO/EP decrease 12.2 and 12.4% in comparison with EP.
Conclusions
NPPh3/DOPO flame-retardant system is applied in epoxy resins. The results show that synergistic effect exists between NPPh3 and DOPO. When the mass ratio of NPPh3 to DOPO is 1:2, the total loading of NPPH3/DOPO is 6%, LOI value of the NPPh3/DOPO/EP system reaches to 33.8% and pass UL-94 V-0 rating. Compared with the thermosets added NPPh3 and DOPO alone in a same addition mass fraction, the LOI value and UL-94 rating all rise. TG test indicates that NPPh3/DOPO greatly promotes the char-forming process and increases the char residue. During combustion, NPPh3/DOPO/EP generates phenol derivatives/water, CO2, and aromatics with quenching effect to gaseous phase. Structure analysis of the combustion residue by FTIR indicts NPPh3/DOPO positively contributes to the condensed phase via the formation of a phosphorus-rich char. This uniform and continuous char layer slow the heat transmission and diffusion, thus protect the underlying materials from further pyrolysis. As a result, the introduction of NPPh3 and DOPO into epoxy resins leads to higher flame-retardant efficiency and thermal stability at a high temperature.
References
Xiao L, Sun D, Niu T, Yao Y. Syntheses and characterization of two novel 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-based flame retardants for epoxy resin. High Perform Polym. 2014;26(1):52–9.
Xu M-J, Xu G-R, Leng Y, Li B. Synthesis of a novel flame retardant based on cyclotriphosphazene and DOPO groups and its application in epoxy resins. Polym Degrad Stab. 2016;123:105–14.
Tan Y, Shao Z-B, Yu L-X, Xu Y-J, Rao W-H, Chen L, Wang Y-Z. Polyethyleneimine modified ammonium polyphosphate toward polyamine-hardener for epoxy resin: thermal stability, flame retardance and smoke suppression. Polym Degrad Stab. 2016;131:62–70.
Huo S, Wang J, Yang S, Wang J, Zhang B, Zhang B, Chen X, Tang Y. Synthesis of a novel phosphorus-nitrogen type flame retardant composed of maleimide, triazine-trione, and phosphaphenanthrene and its flame retardant effect on epoxy resin. Polym Degrad Stab. 2016;131:106–13.
Liu S, Fang Z, Yan H, Chevali VS, Wang H. Synergistic flame retardancy effect of graphene nanosheets and traditional retardants on epoxy resin. Compos A Appl Sci Manuf. 2016;89:26–32.
Fang Y, Zhou X, Xing Z, Wu Y. Flame retardant performance of a carbon source containing DOPO derivative in PET and epoxy. J Appl Polym Sci. 2017;134:12.
Zhang T, Liu W, Wang M, Liu P, Pan Y, Liu D. Synergistic effect of an aromatic boronic acid derivative and magnesium hydroxide on the flame retardancy of epoxy resin. Polym Degrad Stab. 2016;130:257–63.
Müller P, Schartel B. Melamine poly(metal phosphates) as flame retardant in epoxy resin: performance, modes of action, and synergy. J Appl Polym Sci. 2016;133(24). https://doi.org/10.1002/app.43549.
Zhang W, Li X, Yang R. Novel flame retardancy effects of DOPO-POSS on epoxy resins. Polym Degrad Stab. 2011;96(12):2167–73.
Ciesielski M, Schäfer A, Döring M. Novel efficient DOPO-based flame-retardants for PWB relevant epoxy resins with high glass transition temperatures. Polym Adv Technol. 2008;19(6):507–15.
Wang X, Hu Y, Song L, Yang H, Xing W, Lu H. Synthesis and characterization of a DOPO-substitued organophosphorus oligomer and its application in flame retardant epoxy resins. Prog Org Coat. 2011;71(1):72–82.
Yang S, Wang J, Huo S, Cheng L, Wang M. The synergistic effect of maleimide and phosphaphenanthrene groups on a reactive flame-retarded epoxy resin system. Polym Degrad Stab. 2015;115:63–9.
Zhang L, Wang Y, Liu Q, Cai X. Synergistic effects between silicon-containing flame retardant and DOPO on flame retardancy of epoxy resins. J Therm Anal Calorim. 2015;123(2):1343–50.
Zhang W, He X, Song T, Jiao Q, Yang R. The influence of the phosphorus-based flame retardant on the flame retardancy of the epoxy resins. Polym Degrad Stab. 2014;109:209–17.
Chen X, Gu A, Liang G, Yuan L, Zhuo D, Hu J-T. Novel low phosphorus-content bismaleimide resin system with outstanding flame retardancy and low dielectric loss. Polym Degrad Stab. 2012;97(5):698–706.
Yang Y, Luo H, Cao X, Kong W, Cai X. Preparation and characterization of a water resistance flame retardant and its enhancement on charring–forming for polycarbonate. J Therm Anal Calorim. 2017;129(2):809–20.
Bao X, Cai X. Synergistic effect of methyl phenyl silicone resin and DOPO on the flame retardancy of epoxy resins. J Therm Anal Calorim. 2014;118(1):369–75.
Xu M-J, Ma Y, Hou M-J, Li B. Synthesis of a cross-linked triazine phosphine polymer and its effect on fire retardancy, thermal degradation and moisture resistance of epoxy resins. Polym Degrad Stab. 2015;119:14–22.
Zhang X, Zhong Y, Mao Z-P. The flame retardancy and thermal stability properties of poly (ethylene terephthalate)/hexakis (4-nitrophenoxy) cyclotriphosphazene systems. Polym Degrad Stab. 2012;97(8):1504–10.
Xu G-R, Xu M-J, Li B. Synthesis and characterization of a novel epoxy resin based on cyclotriphosphazene and its thermal degradation and flammability performance. Polym Degrad Stab. 2014;109:240–8.
Zhang W, Li X, Li L, Yang R. Study of the synergistic effect of silicon and phosphorus on the blowing-out effect of epoxy resin composites. Polym Degrad Stab. 2012;97(6):1041–8.
Zhang L, Wang Y, Cai X. Effect of a novel polysiloxane-containing nitrogen on the thermal stability and flame retardancy of epoxy resins. J Therm Anal Calorim. 2016;124(2):791–8.
Yang S, Wang J, Huo S, Wang M, Wang J. Preparation and flame retardancy of a compounded epoxy resin system composed of phosphorus/nitrogen-containing active compounds. Polym Degrad Stab. 2015;121:398–406.
Jiao C, Wang H, Li S, Chen X. Fire hazard reduction of hollow glass microspheres in thermoplastic polyurethane composites. J Hazard Mater. 2017;332:176–84.
Zhang W, Li X, Yang R. Pyrolysis and fire behaviour of epoxy resin composites based on a phosphorus-containing polyhedral oligomeric silsesquioxane (DOPO-POSS). Polym Degrad Stab. 2011;96(10):1821–32.
Jiao C, Wang H, Zhang Z, Chen X. Preparation and properties of an efficient smoke suppressant and flame-retardant agent for thermoplastic polyurethane. Poly Adv Technol. 2017;28:1690–1698.
Ding H, Wang J, Wang C, Chu F. Synthesis of a novel phosphorus and nitrogen-containing bio-based polyols and its application in flame retardant polyurethane sealant. Polym Degrad Stab. 2016;124:43–50.
Chen X, Wang W, Li S, Jiao C. Fire safety improvement of para-aramid fiber in thermoplastic polyurethane elastomer. J Hazard Mater. 2017;324(Pt B):789–96.
Xu W, Wirasaputra A, Liu S, Yuan Y, Zhao J. Highly effective flame retarded epoxy resin cured by DOPO-based co-curing agent. Polym Degrad Stab. 2015;122:44–51.
Min Y, Li P, Yin X-G, Ban D-M. Synthesis and characterization of an novel flame retardant based on phosphaphenanthrene for epoxy resin. Polym Bull. 2016;74(1):1–10.
Xie H, Lai X, Li H, Zeng X. Synthesis of a novel macromolecular charring agent with free-radical quenching capability and its synergism in flame retardant polypropylene. Polym Degrad Stab. 2016;130:68–77.
Zhao X, Gao S, Liu G. A THEIC-based polyphosphate melamine intumescent flame retardant and its flame retardancy properties for polylactide. J Anal Appl Pyrol. 2016;122:24–34.
Acknowledgements
We would like to thank the generous supports by the following: the experiment center of polymer science and engineering academy, Sichuan University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, H., Zhou, F., Yang, Y. et al. Synergistic flame-retardant behavior and mechanism of tris(3-nitrophenyl) phosphine and DOPO in epoxy resins. J Therm Anal Calorim 132, 483–491 (2018). https://doi.org/10.1007/s10973-017-6898-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6898-8