Abstract
A novel polysiloxane (HPS) with epoxy and phenyl groups was synthesized by controlled hydrolysis and condensation of γ-(2,3-epoxypropoxy)propytrimethoxysilane (KH560) and diphenyl silanediol. Besides, HPS was used as the compatibilizer of the miscible diglycidyl ether of bisphenol A (DGEBA)/methyl phenyl silicone resin (Si603) blend. The structure and effect of HPS were characterized by Fourier transform infrared spectra, nuclear magnetic resonance (1H-NMR), differential scanning calorimetry, and scanning electron microscopy (SEM). The results showed that HPS could significantly improve the compatibility between epoxy resin (EP) and Si603 resin. In addition, the glass transition temperature (T g) of the blend increases with increasing amount of Si603 from 129 to 151 °C. The thermal stability of blending system was studied by thermogravimetric analysis, derivative thermogravimetric analysis and SEM. The results showed that the incorporation of Si603 into DGEBA resin not only obviously increased the thermal resistance, but also remarkably improved the flame retardancy. The high limiting oxygen index of the HPS/EP/Si603/DDM system at 31 is considered as excellent flame retardancy in the epoxy system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, developing high-performance resins are widely used in science and engineering, owing to their outstanding integrated properties and great importance in many cutting-edge fields, especially aerospace, electric and electronic industries [1–3]. For example, among the common engineering polymers, epoxy resins have occupied a dominant position in the high performance materials. Epoxy resins are widely used as coatings, adhesives, laminates, semiconductor encapsulation, and matrices for advanced composites, because of their outstanding mechanical stiffness, toughness, good solvent, chemical resistance, and superior adhesion [4–6]. However, the poor flammability of epoxy resins is a major disadvantage in special applications. Many techniques have been used to improve the flame retardancy of epoxy resins. Due to environmental problems, the use of non-halogenated flame retardants shall become main stream in the future. Among them, the silicone-containing epoxy flame retardants will be one of the choices.
Polysiloxane-modified epoxy resins possess good thermal stability and flame resistance. So it has been used in polymer modification [7]. There is no doubt that the polysiloxane modified epoxy resin is a very effective way to impart good thermal stability and flame resistance in polymer modification. For these polysiloxane modified epoxies [8], the conversion to the stable silicon dioxide in air can form a glassy layer on polymer surfaces to cut off the heat and oxygen transfer and improve the flame retardation of the polymers. But this system contains large amounts of oxygen, leading to phase separation and substantial lower glass transition temperatures [9]. If the substitution group is a phenyl group, the glass transition temperature is better than that of an alkyl group.
In this research, methyl phenyl silicone resin (Si603) was selected as adding material to modify epoxies. Because Si603 resin contains a lot of phenyl, high thermal stability and carbon residue. But they possess very poor compatibility. In order to achieve good results, we also used other compatibilizers. Such as, organic silicon only contains epoxy-groups or amine-groups. However, they did not reach the goal. Therefore, we have synthesized a hyperbranched polymer that contains the epoxy group and phenyl. The hyperbranched polymer presents the similar structures with both the epoxy and silicone resin to improve the compatibility between epoxy resins and silicone resin. Compared with their linear analogs, hyperbranched polymers possess considerably lower viscosity [10]. In addition, the existence of many functional groups provides great possibilities for chemical modifications [11]. In recent years, hyperbranched polymers have been successfully applied to toughen thermosetting resins. For example, Zhang’s group toughened EP resin with hyperbranched polyimide [12], Xu’s group toughened bismaleimide resin with hyperbranched polyester [13].
In this paper, we used hyperbranched polymer to modify the compatibility. The compatibilization, thermal degradation behavior, and the flame retardant effect were investigated by scanning electron microscopy (SEM), Differential scanning calorimentry (DSC), Thermogravimetric analysis (TG), and limiting oxygen index (LOI).
Experimental
Materials
Diglycidyl ether of bisphenol A (DGEBA, EP, epoxy value = 0.44 mol/100 g) was supplied by Wuxi Bluestar Chemical Co. Ltd. China. Distilled water, 4,4-diaminodiphenylmethane (DDM), acetic acid (CH3COOH), and anhydrous ethanol were obtained from Chengdu Kelong Chemical Reagent Factory. Methyl phenyl silicone resin (Si603) was supplied by WACKER. Germany. γ-(2,3-Epoxypropoxy)propytrimethoxysilane (KH560) and diphenylsilanediol were supplied by Jinan Yijia Chemical Co. Ltd. China.
Synthesis of polysiloxane (HPS)
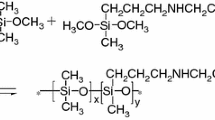
A 250 mL three-necked round bottom flask equipped with a stirrer, a thermometer, and a condenser was charged with 32.4 g diphenylsilanediol and appropriate mass of anhydrous ethanol. Then 25 g KH560, 2 g H2O were added until diphenylsilanediol dissolves completely. After that, a spot of CH3COOH was added slowly into the flask as catalyst. After being maintained at room temperature for 20 min, the solution was heated to 60 °C with stirring for 4.5 h. Finally, the product was put into a vacuum oven to remove methanol, ethanol, and water. Thereafter, a transparent and viscous liquid was obtained, which is hyperbranched polysiloxane (coded as HPS). The reaction mechanism of this system is shown in Fig. 1.
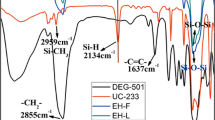
FTIR (KBr) (cm−1): 3423 (Si–OH), 1042-1138 (Si–O-Si), 1592 and 1429 (aromatic protons), 910 (oxirane ring) as indicated in Fig. 2. 1H–NMR (400 MHz, CDCl3) is indicated in Fig. 3.
Determination of the epoxy value of polysiloxane (HPS)
The epoxy value of the epoxy compound was determined with titration. 1.0 g epoxy compound was put into a 150 mL conical flask, and then 25 mL HCl/acetone (volume fraction 2.44 %) solution was added. After the epoxy compound dissolves in the HCl/acetone solution totally, the mixture was placed for one hour. Then 0.2 mL phenolphthalein solution (0.1 mass% in methanol) was added into the system as indicator. The solution was titrated with a NaOH solution (0.1 N in methanol). The epoxy value of the epoxy compound was determined with E = (V 1−V 2)*C/(10 M). Experiment shows that test value (E = 0.12 mol/100 g) is less than the theoretical value (E = 0.17 mol/100 g), because of ring opening reaction.
Where M is the mass of epoxy compound (g), V 1 is the average value of 0.1 N NaOH solution for two blank tests (mL), V 2 is the used amount of 0.1 N NaOH solution for sample titration (mL), and C is the NaOH solution’s concentration.
Preparation of cured HPS/EP/Si603/DDM blends
Various composition mixtures of HPS/EP/Si603n/DDM were cured by DDM as the following steps. First, appropriate amounts of HPS and epoxy with a ratio at 0.05:1 (mass) were blended by stirring at 130 °C for 20 min. Second, the appropriate amount of Si603 was put into the prepolymer above. Third, stoichiometric DDM was casted into the blend above to dissolve at 100 °C. Subsequently, the blend was put into a mold for curing and postcuring at oven as the following procedure: 80 °C/2 h + 150 °C/3.5 h +165 °C/1.5 h.
Instrumentation
FTIR spectroscopy was applied with a Nicolet IS10 FTIR spectrometer using KBr chip. 1H NMR (400 Hz) spectra were recorded on a FT-80A NMR using CDCl3 as a solvent. The Netzsch Q-200 was used for Differential scanning calorimentry (DSC) under nitrogen atmosphere using a scanning rate of 10 K min−1. The morphologies of the fractured surfaces of samples and the surface morphology of the char obtained after the LOI test were observed using Inspect-F SEM. Thermogravimetric analysis (TG) tests were carried out by the TG (Netzsch TG209) at a linear heating rate of 10 K min−1 under pure nitrogen and air within the temperature range from 30 to 800 °C. LOI data of all samples were obtained at room temperature on an oxygen index instrument (XYC-75) produced by Chende Jinjian Analysis Instrument Factory, according to GB/T2406-93 standard. The dimensions of all samples were 130 × 6.5 × 3 mm3. LOI was an important parameter for evaluating the ease of extinguishment of polymeric materials in the same condition. It denotes the lowest volume concentration of oxygen sustaining candle burning of materials in the mixing gases of nitrogen and oxygen.
Results and discussion
Characteristics of the HPS
In order to confirm the molecular structure of HPS, FTIR, 1H-NMR analyses were carried out. Figure 2 is the FTIR spectrum of HPS. It can be seen that there is a strong and wide bending vibration peak of Si–O–Si group at 1,036–1,165 cm−1. These suggest that alkoxy groups are successfully changed into Si–O–Si through the hydrolysis. But a stretching vibration peak of Si–O–C group at 1,180 cm−1 states that there are residual methoxyl groups. Meanwhile, there are vibration peaks of the phenyl at 1,429 and 1,592 cm−1 to indicate the existence of the phenyl in HPS. The characteristic peak of Si–OH at 3,423 cm−1 shows that Si–OH groups in the raw material do not completely disappear during the hydrolysis. The reason contributing to the phenomena is that the existence of γ-(2,3-epoxypropoxy)propytrimethoxy and phenyl leads to big steric effect on the polymerization of Si–OH with Si–OH. This similar conclusion was also obtained by Zhang et al. [14]. We also see that there is a vibration peak of oxirane ring group at 910 cm−1. So the polymerization between KH560 and diphenylsilanediol occurred.
So as to further determine the structure of HPS. Figure 3 shows the 1H-NMR spectrum of HPS (ppm): 2.6 (a), 2.79 (b), 3.18 (c), 3.67 (d), 3.32 (e), 3.48 (f), 1.62 (g), 3.52 (k), 0.67 (i), 7.12-7.66 (j) [15]. Experiments show that the chemical shifts match those of the chemical structure of the desired product. These suggest that hydrolysis is successfully processed.
Compatibility of cured HPS/EP/Si603/DDM blends
The influence of HPS on the morphologies of HPS/EP/Si603n/DDM blend can be evaluated by the glass transition temperature (T g) peak. It is known that an absolutely miscible blend presents only one T g peak. But the peak of each phase of an immiscible blend will be observed and the peak of each phase will shift toward the same point with the improvement of the compatibility [16, 17].
Figure 4 gives DSC curves of various cured EP/DDM, EP/6 %Si603/DDM and HPS/EP/Si603n/DDM mixtures. Except EP/6 %Si603/DDM, all the curves from mixtures have a single glass transition temperature (T g) peak between 129 and 153 °C in Table 1. It can be seen that the 5 %HPS/EP/DDM mixture gives the lowest T g (129 °C), but the EP/DDM system gives the biggest T g (153 °C). The rest of the mixed epoxy systems also exhibit single T g between 129 and 153 °C in Fig. 4. The more Si603 the content is, the higher the T g is. The phenomenon can be mainly interpreted by two facts. First, the chemistry of 5 %HPS/EP/DDM resins is different from that of EP/DDM resin. The former has additional flexible and tough structure due to the large free volume, which was caused by a small amount of Si–C6H5 and Si–O–Si groups in HPS. The phenyl on the side chain expands the distance between the molecular chains and reduces the crosslink density. So the cross-linking density of the HPS/EP/DDM resins is lower than that of EP/DDM resin [18]. But the latter has rigid chain. With the increasing of the content of Si603 resin, the T g becomes more and more higher. This is because that Si603 resin contains much Si–C6H5. A large number of phenyl increase the chain rigidity and prevent the movement of the chain. This single T g phenomenon indicates a homogeneous morphology. But EP/6 %Si603/DDM has two glass transition temperature (T g) peaks at 73 and 154 °C, which belong to Si603 resin and EP/DDM in Table 1. This suggests that the epoxy resin and Si603 occurred phase separation.
In order to further illustrate the influence of HPS. The SEM morphologies of the fractured surfaces of samples are shown in Fig. 5. We can see that cured 5 %HPS/EP/6 %Si603/DDM resin is uniform and transparent yellow. It can be seen that the fractured surface is flat. There are a lot of balls and circular holes on the fractured surface of sample in Fig. 5a. They are more evenly distributed in epoxy matrix. The fractured surface of 5 %HPS/EP/6 %Si603/DDM was covered in anhydrous ethanol for 20 h. There are a large number of circular holes on the surface of materials in Fig. 5b. This is due to balls that are made up of Si603 resin dissolved in anhydrous ethanol and leaved the circular holes. But EP/6 %Si603/DDM resin is laminated from the sample. Bottom is translucent yellow substance and the upper is white opaque. Figure 5c is the SEM of EP/6 %Si603/DDM. We hardly watch balls and holes in the fractured surface of sample. Due to Si603 resin migrated to the surface of the matrix in the process of blend and curing. Moreover, reunite also appear in blending process. These phenomena owe to the poor compatibility between EP and Si603 resin. These demonstrate that the EP/Si603/DDM has serious phase separation.
It is interesting to note that when the content of HPS remains the same, all the HPS/EP/Si603n/DDM resins are transparent and homogeneous with the increasing of the content of Si603 resin. All these above illustrates HPS greatly improves the compatibility of this system.
Flammability
The flame retardant property of cured EP/DDM and HPS/EP/Si603n/DDM resins was evaluated by measuring their LOI values. Data are listed in Table 2. With Si603 resin incorporation, the LOI values of cured HPS/EP/Si603n/DDM resins were leveled up from 22 to 31. It can be clearly seen that the LOI values increase rapidly with the Si603 resin increasing. At the same time, Si603 resin will produce less smoke in the combusting process. Meanwhile, the mixtures with Si603 resin can keep the original shape and form hollow shell after burning.
The SEM micrographs of the char residue of EP/DDM and 5 %HPS/EP/12 %Si603/DD±M are shown in Fig. 6. The char residue for SEM test was obtained by burning thoroughly in air. Figure 6a is the SEM micrograph of 5 %HPS/EP/12 %Si603/DDM. It was found that the charred layer was smooth and compact. There was little flaw and lots of tight ball that can increase the thickness and density of carbon layer on the surface of the charred layer, resulting in insulating oxygen very well. Figure 6b is the SEM micrograph of EP/DDM. The surface was rough and there were flaw on the surface of the charred layer. As a result, oxygen ca not be isolated and this does not effectively prevent heat and matter transfer.
Thermal analysis
The thermal stability of cured EP/DDM and HPS/EP/Si603n/DDM resins were characterized by TG under nitrogen and air atmosphere. The typical data obtained from TG and DTG curves. T 5 % is usually used to evaluate the thermal degradation and thermal stability of materials [19].
Figure 7 shows the TG and DTG curves of cured EP/DDM and HPS/EP/Si603n/DDM resins in nitrogen atmosphere. It can be observed that all resins have one-stage decomposition process. The thermal degradation of HPS/EP/Si603n/DDM resins also has one-stage decomposition which is similar with cured EP/DDM. The T 5 %s (the initial decomposition temperature) of HPS/EP/DDM and HPS/EP/Si603n/DDM resins is lower than that of EP/DDM in Table 3. Due to the initial decomposition of HPS occurs at inferior temperature. The Si–O group of HPS is able to absorb more thermal energy and its vibration can dissipate the thermal decomposition energy [20]. The temperature of the maxima mass loss rate (T max) of HPS/EP/DDM is higher than that of EP/DDM. This is caused by the phenomenon that the decomposition of HPS at inferior temperature leads to the formation of the silicone-containing group, which will participate in the crosslinked carbonization. It can convert the usual organic decomposition to partially inorganic decomposition by forming the carbon-silicon residue to act as thermal insulation [8, 21, 22] and to prevent gas evolution, and achieve ultimate improvement on flame retardation of this silicone-containing epoxy system. Meanwhile, with the increasing of the content of Si603 resin, T max of HPS/EP/Si603n/DDM resins is almost the same.
Figure 8 shows the TG and DTG curves of cured EP/DDM and HPS/EP/Si603n/DDM resins in air atmosphere. We can see that all resins have two decomposition stages. Bits of decompositions appear before 300 °C, those are mainly attributed to the decomposition of EP and its derivates in EP matrix. These decompositions indicate that the alkyl groups in the hybrids are not stable. Thus the chain scission of the isopropylidene linkage takes place, resulting in the release of EP and its derivates at the initial decomposition stage [23]. For EP/DDM resin, the first thermal decomposition is occurred between 362 and 470 °C, the second stage is from 470 to 700 °C. But the first thermal decomposition of the other resins, occurring between 350 and 500 °C, is wider than that of EP/DDM resin, and the second stage is from 500 to 700 °C. T maxs of HPS/EP/Si603n/DDM resins become higher, especially the T max2, with the content of Si603 resin increasing. Such as, T max2 of 5 %HPS/EP/12 %Si603/DDM is about 47 °C higher than that of EP/DDM resin in Table 3. Those indicate that Si603 resin can form more effective barrier layer to protect underlying hybrids from decomposing at high temperature. This is because that Si603 resin has much oxydiphenylsilane group and oxypheny methylsilane group. We all know that oxydiphenylsilane group (-O-SiPh2-O-) was reported to exhibit a high thermal stability over 400 °C [24–26]. It was reasonable that replacing triglycidyloxyphenylsilane group with oxydiphenyl- silane group (-O-SiPh2-O-) significantly improved the silicon-containing epoxy resins’ thermal stability. We can also see that Si603 resin have high thermal stability from the TG curve of Si603 resin. Over 400 °C, Si603 resin appears decomposition and the solid char yield in nitrogen and air at 800 °C is 70 and 40 %. These instruct that thermal stability of Si603 resin is significantly higher than that of other resins.
Figure 9 shows experimental and theoretical TG curve of 5 %HPS/EP/6 %Si603 system in N2 and air. All curves are similar and the actual carbon residue rate is higher than the theoretical value in N2 or air. The addition of organic silicon improves the amount of carbon residue. This is because that organic silicon generated more stable substance at high temperatures. This kind of material, which likes isolation belt traps the flammable small molecule diffusion and heat transfer to prevent the underlying substance further decomposed and increase the amount of carbon residue. This effectively improves the thermal stability of the matrix.
Higher char yield from Si603 resin indicates that the carbonization mechanism indeed plays an important role in flame retardation. It is well known that the general flame retarding mechanism of organic silicone resin-based materials is thought to be the formation of protective barrier during combustion. The improved flame retardance can be attributed to a flame retarding mechanism, including following flame retarding effects: improving thermal stability, acting in the condensed phase and providing a barrier for heat, and mass transfer [27].
Conclusions
A novel polysiloxane (HPS) with epoxy and phenyl groups is synthesized by controlled hydrolysis of γ-(2,3-epoxypropoxy)propytrimethoxysilane (KH560) and diphenylsilanediol. Experiments show that HPS can significantly improve the compatibility between EP and Si603 resin. Specifically, the thermal stability of the blending system with Si603 resin was improved. The glass transition temperature and SEM micrograph of HPS/EP/Si603n/DDM resins show homogeneous distribution. The incorporation of Si603 resin can also increase the flame retardation effect. The LOI value of the EP can attain 31 when Si603 resin was added.
References
Fu SY, Zheng B. Templated silica tubes with high aspect ratios as effective fillers for enhancing the overall performance of polyimide films. Chem Mater. 2008;20:1090–8.
Wang DM, Zhang JH, Lin Q, Fu LS, Zhang HJ, Yang B. Lanthanide complex/polymer composite optical resin with intense narrow band emission, high transparency and good mechanical performance. J Mater Chem. 2003;13:2279.
Tang HY, Li WW, Fan XH, Chen XF, Shen ZH, Zhou QF. Synthesis, preparation and properties of novel high-performance allyl–maleimide resins. Polymer. 2009;50:1414.
Gojny FH, Wichmann MHG, Fiedler B, Kinloch IA, Bauhofer W, Windle AH, Schulte K. Evaluation and identification of electrical and thermal conduction mechanisms in carbon nanotube/epoxy composites. Polymer. 2006;47:2036–45.
Wetzel B, Haupert F, Qiu Zhang M. Epoxy nanocomposites with high mechanical and tribological performance. Compos Sci Technol. 2003;63:2055–67.
Rosu D, Cascaval CN, Mustata F, Ciobanu C. Cure kinetics of epoxy resins studied by non-isothermal DSC data. Thermochim Acta. 2002;383:119–27.
Smith SD, Long TE, McGrath JE. Thermogravimetric analysis of poly(alkyl methacrylates) and poly(methylmethacrylate-g-dimethyl siloxane) graft copolymers. J Polym Sci Part A. 1994;32:1747–53.
Kambour RP, Klipfer HJ, Smith SA. Limiting oxygen indices of silicone block polymers. J Appl Polym Sci. 1981;26:847.
Lin ST, Huang SK. Synthesis and characterization of siloxane-modified epoxy resin. J Polym Res. 1994;1:151.
Amerio E, Sangermano M, Malucelli G, Priola A, Rizza G. Preparation and characterization of hyperbranched polymer/silica hybrid nanocoatings by dual-curing process. Macromol Mater Eng. 2006;91:1287–92.
Mahapatra S, Singha KN. Hyperbranched polyamine/cu nanoparticles for epoxy thermoset. J Mater Sci Part A. 2009;46:296–303.
Jin FL, Park SJ. Thermal properties and toughness performance of hyperbranched-polyimide-Modified epoxy resins. J Polym Sci, Part B. 2006;44:3348–56.
Xu J, Wu H, Mills OP, Heiden PA. A morphological investigation of thermosets toughened with novel thermoplastics. I. Bismaleimide modified with hyperbranched polyester. J Appl Polym Sci. 1999;72:1065–76.
Zhang GB, Fan XD, Liu YY, Kong J, Wang SJ. Structure design of novel hyperbranched polycarbosilazanes: synthesis and characterization. J Polym. 2007;7:644–6.
Torry SA, Campbell A, Cunliffe AV, Tod DA. Kinetic analysis of organosilane hydrolysis and condensation. Int J Adhes Adhes. 2006;26:40–9.
Couchman P. Compositional variation of glass-transition temperatures. 2. Application of the thermodynamic theory to compatible polymer blends. Macromolecules. 1978;11:1156–61.
Wang WJ, Perng LH, Hsiue GH, Chang FC. Characterization and properties of new silicone-containing epoxy resin. Polymer. 2000;41:6113–22.
Sun Bin, Liang Guozheng, Aijuan Gu, Yuan Li. High performance miscible polyetherimide/bismaleimide resins with simultaneously improved integrated properties based on a novel hyperbranched polysiloxane having a high degree of branching. Ind Eng Chem Res. 2013;52:5054–65.
Gu AJ. Thermal degradation behaviour and kinetic analysis of Epoxy/montmorillonite nanocomposites. Polym Degrad Stab. 2003;80:383–91.
Kanai H, Sullivan V, Auerback A. Impact modification of engineering thermoplastics. J Appl Polym Sci. 1994;53:527.
Kambour RP. Flammability resistance synergism in BPA polycarbonate–silicone block polymers. J Appl Polym Sci. 1981;26:861.
Kambour RP, Ligon WV, Russell RP. Enhancement of the limiting oxygen index of an aromatic polycarbonate by the incorporation of silicone blocks. J Polym Sci, Part C. 1978;16:327.
Curry JE, Byrd JD. Silane polymers of diols. J Appl Polym Sci. 1965;9:295.
Wang X, Hu Y, Song L, Xing W, Lu H, Lv P, Jie G. Flame retardancy and thermal degradation mechanism of epoxy resin composites based on a DOPO substituted organophosphorus oligomer. Polymer. 2010;51:2435–45.
Dunnavant WR, Markle RA, Curry JE, Byrd JD. Synthesis of polyaryloxysilanes by melt-polymerizing dianilino-and diphenoxysilanes with aromatic diols. J Polym Sci Polym Chem Ed. 1967;5:707.
Liaw DJ, Liaw BY. Synthesis and characterization of novel polyaryloxydiphenylsilane derived from 2, 2-dimethyl-biphenyl-4,4-diol. J Polym Sci Part A. 1999;37:4591.
Wu CS, Liu YL, Chiu YS. Epoxy resins possessing flame retardant elements from silicon incorporated epoxy compounds curing with phosphorus or nitrogen containing curing agents. Polymer. 2002;43:4277–84.
Acknowledgements
We would like to thank the generous financial support by the following grant: National Natural Sciences Foundation of China, Grant no. 50973066.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Q., Bao, X., Deng, S. et al. The investigation of methyl phenyl silicone resin/epoxy resin using epoxy-polysiloxane as compatibilizer. J Therm Anal Calorim 118, 247–254 (2014). https://doi.org/10.1007/s10973-014-4017-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-4017-7