Abstract
In the present work, new data on the densities and refractive indices for 1-hexyl-3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate [hmim][FAP] with N-methyldiethanolamine are reported for various concentrations and at temperatures (303.15–328.15) K. Excess molar volumes \({V^{\text{E}}}\) and excess refractive indices \( n_{\text{D}}^{\text{E}}\) were calculated from the experimental data. Refractive index values for the binary mixtures were predicted by using Lorentz–Lorenz, Gladstone–Dale and Eykman equations. Excess molar volumes showed positive trend, whereas excess refractive indices showed negative trend over the entire range of temperatures and concentrations studied in the present research.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ionic liquids (ILs) are organic salts having melting points less than 100 °C. Ionic liquids consist of large heterocyclic organic cations and inorganic or organic anions. The most commonly available/used cations are: N-alkylpyridinium, tetraalkylammonium, tetraalkylphosphonium and imidazolium. The anionic part could be halides, acetate, nitrate, tetrafluoroborate ([BF4]), hexafluorophosphate ([PF6]), trifluoromethylsulfonate ([OTf]), bis(trifluoromethylsulfonyl)imide ([Tf2N), tris(pentafluoroethyl)trifluorophosphate ([FAP]) and the others [1, 2]. ILs have garnered much attention over the past decade due to their unique properties, e.g., low vapour pressure, non-flammability, non-volatility, easily modified structure, good solvency power for organic and inorganic compounds and very high thermal stability [3, 4]. These properties make ILs as good candidates and promising green alternative solvents for a number of industrial applications. Some examples of commercial applications of ILs include usage as operating fluid in several electrochemical applications (batteries, photovoltaic cell, capacitors, fuel cells etc.), as lubricants, as biomass-processing fluids, as heat transfer fluids, as reaction solvents, as catalysts, as separating agents in azeotropic and extractive distillation and as absorption media for gasses [5]. Application of ionic liquids towards carbon dioxide capture has recently been studied and reported by many researchers [6–9]. Currently, aqueous amine solutions are being used extensively and effectively (due to their reactivity) in industry for CO2 removal. However, usage of amine solutions is an energy-extensive and unfriendly process towards environment; furthermore, amine solutions cause corrosiveness to the process equipments [10]. Hence, development of environmentally benign and energy-intensive solvents which are capable of removing CO2 efficiently is the current focus of the researchers. ILs are envisaged as potential green alternative solvents for CO2 capture. However, large-scale industrial usage of ILs for the said purpose is not viable due to their high cost of synthesis and high viscosity. Therefore, scientists’ pursuit for environment-friendly, energy-efficient solvents (to capture CO2) which are industrially applicable also is still going on. Recently, hybrid solvents comprising of ILs and amines have been explored by many researchers as alternative solvents for CO2 capture [11, 12]. These hybrid solvents are expected to possess the environmental-friendly characteristics of ILs coupled with the reactivity of amines. Therefore, highly viscous and highly priced ILs could be mixed with cheap and low-viscous amines to form different combinations of usable binary mixtures for CO2 capture. Camper et al. [13] put forward the idea of mixing amines with ILs to form mixtures, used for efficient and reversible CO2 capture. It was reported that attempted mixtures exhibited better CO2 capture ability as compared to amine functionalized ILs. In the same manner, Feng et al. [14] studied the solubility of CO2 in mixtures of four ILs with N-methyldiethanolamine (MDEA). It was observed that mixtures of ILs with amines showed superior performance (in terms of CO2 solubility) than the aqueous amine solutions. It has been reported that knowledge of physical properties data pertaining to the solvent used for CO2 capture is of utmost importance as it aids in designing and scaling up of process equipments. Physical properties data also help to envision the use of certain solvents for a particular application.
It has been reported that imidazolium-based ILs are much lucrative for CO2 capture [7]. On the other hand, among the available amines, N-methyldiethanolamine (MDEA) is noticeable due to some of its unique characteristics like high thermal and chemical stability, low vapour pressure and less corrosive behaviour [15]. Therefore, in the present work, binary mixtures of 1-hexyl-3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate [hmim][FAP] with N-methyldiethanolamine (MDEA) were prepared at different mole fractions. These binary mixtures could be potential solvents for CO2 capture, and in order to characterize the prepared mixtures, their physical properties have been studied. Density ρ and refractive indices n D for the binary mixtures were measured at different mole fractions and at temperature range of (303.15–328.15) K. Based on the experimental data, excess molar volumes \({V^{\text{E}}}\) and excess refractive indices \({n_{\text{D}}^{\text{E}}}\) were calculated. Refractive indices values for the binary mixtures were predicted theoretically by using the equations proposed by Lorentz–Lorenz, Gladstone–Dale and Eykman.
Experimental
Materials
All the chemicals were purchased from Merck Chemicals. Ionic liquid, 1-hexyl-3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate [hmim][FAP], was supplied with a purity of ≥99 % (determined by HPLC). The structure of [hmim][FAP] is shown in Fig. 1. [hmim][FAP] was dried under vacuum at 80 °C for 48 h before use. N-methyldiethanolamine (MDEA) was supplied with a stated purity ≥99.5 % (GC, area %) and used as received.
Water and chloride content
The water content of [hmim][FAP] was determined by using Karl Fischer coulometric titrator (Mettler-Toledo, DL39) with Hydranal coulomat AG reagent (Riedel-de-Haen). The water content of [hmim][FAP] used in this study was 298 ppm. The chloride content of dried [hmim][BF4] was determined by using DL-55 autotitrator (Mettler-Toledo) with 0.005 M, AgNO3 as the titrant. The estimated chloride content was 34 ppm. The water content of MDEA used in this study was determined by Karl Fischer titrator (Mettler-Toledo, DL-39) using Hydranal coulomat E reagent (Riedel-de-Haen) with benzoic acid (90 mL anolyte + 5 g benzoic acid) as standard procedure described for amines in Mettler-Toledo (DL-39) operating manual. The water content thus estimated was 2768 ppm.
Apparatus and procedure
All the samples were freshly prepared and retained at desired temperature for 24 h to ensure complete solubility. The samples were prepared on mass basis by using analytical balance (Mettler-Toledo, model AS 120S) and later converted to mole fractions. Uncertainty in the mole fraction calculations was estimated to be around ±0.0001. Binary mixtures were prepared in glass vials and closed with screw caps fitted with PTFE septum. The samples were taken out with a syringe and immediately placed into the apparatus for each measurement to avoid the humid effects.
Density measurement
The densities of all the binary mixture as well as pure MDEA and [hmim][BF4] were measured by using oscillating U-tube density meter (Anton Paar, DMA-5000) at temperatures (303.15–328.15) K with an uncertainty of ±0.01 K. The density meter was calibrated by using Millipore water and dry air [16]. For the validation of calibrated density meter, pure liquids of known density, namely [bis(2-hydroxyethyl)ammonium acetate, water, monoethanolamine and bis(2-hydroxyethyl)ammonium acetate], were used [16, 17]. The uncertainty of all measurements was better than 3 × 10−5 g cm−3, and the overall precision in experimental density measurements was better than ±1 × 10−5 g cm−3.
Refractive index measurement
Refractive indices of all the binary mixture samples as well as pure liquids were measured at temperatures (303.15–328.15) K by using ATAGO programmable digital refractometer (RX-5000 alpha) with a temperature control accuracy of ±0.05 °C. The apparatus was precise to within 2 × 10−5 and the uncertainty of the measurement was better than ±4 × 10−5. The apparatus was calibrated by using Millipore water and the measurements were validated by measuring the refractive index of pure liquids (methanol, 1-butyl-3-methylimidazolium tetrafluoroborate) whose values have been already reported [17, 18].
All the densities and refractive indices measurements for each sample were performed in triplicate, and the average values are reported and considered for further analysis.
Results and discussions
In this present work, new data on the densities and refractive indices for 1-hexyl-3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate [hmim][FAP] with N-methyldiethanolamine (MDEA) are reported for various concentrations and at temperatures (303.15–328.15) K. Excess molar volumes V E and excess refractive indices \( n_{\text{D}}^{\text{E}}\) were calculated from the experimental data. Refractive index values for the binary mixtures were predicted by using Lorentz–Lorenz, Gladstone–Dale and Eykman equations. Table 1 compare the measured densities and refractive indices values of pure 1-hexyl-3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate [hmim][FAP] and N-methyldiethanolamine (MDEA) with the available literature data. The measured values were found to be in good agreement with the literature values [19–23]. The present measured values of densities and refractive indices for [hmim][FAP] with N-methyldiethanolamine (MDEA) are listed in Table 2. It is evident that [hmim][FAP] has higher density as compared to MDEA. For binary mixtures, the density values increased as the mole fraction of [hmim][FAP] increased. The density values of binary mixtures as well as pure liquids (MDEA, [hmim][FAP]) decreased with increase in temperature as expected (Table 2). Based on this analysis, the measured density values can be expressed as a function of temperature and concentration simultaneously by using the following form of expression [18]:
where x is the mole fraction of [hmim][FAP], T is the temperature in Kelvin and A i, B i, C i are the correlation coefficients obtained by regression. The estimated correlation coefficients are presented in Table 3 along with the standard deviation (σ) values obtained by using the following relation:
where Zexp is the experimental value, Zcal is the calculated value and ‘n’ is the number of experimental data points.
Excess molar volumes V E for the binary mixtures were calculated from the measured density data by employing the following relation [24]:
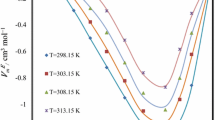
where ρ is the density of the binary mixture; x 1 and x 2 are the mole fractions of pure component 1 and 2, respectively; M 1 and M 2 are the molar masses of pure components 1 and 2, respectively; ρ 1 and ρ 2 are the densities of the pure components 1 and 2, respectively. The calculated values of excess molar volumes are listed in Table 2. Excess molar volume values showed positive trend over the entire range of temperatures and concentrations (Fig. 2). It has been observed that excess molar volume is the resultant of several opposing effects. These effects can arbitrarily be divided into three types: chemical, physical and structural. Physical contributions which are non-specific forces of interaction, like dispersion forces or weak dipole–dipole interactions (due to hydrogen bond rupture) between unlike molecules in the mixtures, lead to positive values of V E. Meanwhile, positive values of excess molar volumes are also attributed to the structural breaking effects [25–29]. Positive values of V E increased with increase in mole fraction of [hmim][FAP] and reached a maximum value at x 1 = 0.5999. After that point with increase in mole fraction of [hmim][FAP], the positive values of V E decreased (Fig. 2). The values of V E changed predominantly with variation in concentration of either pure component ([hmim][FAP] or MDEA). In [hmim][FAP]-rich areas (x 1 = 0.8003, 0.8993) or in MDEA-rich areas (x 1 = 0.1007, 0.1989), excess molar volume values were less positive indicating the ability of solvents to oppose the structural breaking effects, while one is in high concentration as compared to the other constituent comprising the mixture. Refractive index values were higher for MDEA as compared to [hmim][FAP]. In binary mixtures the refractive index values increased as the mole fraction of [hmim][FAP] decreased. Refractive index values decreased with increasing temperature, as expected (Table 2). Variations in the refractive indices values with change in temperatures and concentrations are appreciable, and hence an attempt has been made to correlate refractive indices as a function of temperature and concentration simultaneously by using Eq. 1. The estimated fitting parameters are presented in Table 3.
Excess molar volume V E versus mole fraction x 1 for the system [hmim][FAP] (1) + MDEA (2), at several temperatures: diamond 303.15 K; square box 308.15 K; triangle 313.15 K; cross sign 318.15 K; asterisk 323.15 K; circle 328.15 K. The solid lines were calculated by ρ calc. using Eq. 1
Refractive index values of the binary mixtures were predicted theoretically by using the equations proposed by Lorentz–Lorenz (Eq. 4), Gladstone–Dale (Eq. 5) and Eykman (Eq. 6).
In the above equations, n D, is the refractive index of the mixture; φ i and n Di are the volume fraction and refractive index of the component i, respectively. The deviations between the experimental values of refractive indices and the one calculated with the help of above equations are listed in Table 4. The calculated values of refractive indices with Lorentz–Lorenz, Gladstone–Dale, and Eykman equations agreed well with the experimental values as the deviation was within the allowable limit (≤0.005). In order to calculate the ideal refractive index (n idD ) and excess refractive index (n ED ) of the binary mixtures ([hmim][FAP] with N-methyldiethanolamine (MDEA)) the equations proposed by Reis et al. [30] were used. The ideal refractive index is expressed as:
where φ 1 and φ 2 are the volume fractions of pure component 1 and 2 and n D1 and n D2 are the refractive index of pure component 1 and 2, respectively. The excess refractive index was calculated by using the following expression.
where \( n_{\text{D}}^{\text{E}} \) is the excess refractive index and n D is refractive index of mixtures and \( n_{\text{D}}^{\text{id}}\) is the ideal refractive index calculated using Eq. (7). Excess refractive indices values showed negative trend over the entire range of temperatures and compositions (Fig. 3).
Excess refractive indices n ED for the system [hmim][FAP] (1) + MDEA (2), at several temperatures; diamond 303.15 K; triangle 308.15 K; asterisk 313.15 K; plus 318.15 K; minus 323.15 K; filled square, 328.15 K. The solid curves were calculated by n Dcalc using Eq. 1
Conclusions
Binary mixtures of 1-hexyl-3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate [hmim][FAP] with N-methyldiethanolamine (MDEA) were prepared at different mole fractions. The densities and refractive indices of the binary mixtures were measured at a temperature range of (303.15–328.15) K. The densities of the present prepared binary mixtures increased as the mole fraction of [hmim][FAP] increased. Refractive index values were higher for MDEA as compared to [hmim][FAP]. In mixtures the refractive index values increased as the mole fraction of [hmim][FAP] decreased. Excess molar volumes showed positive values over the entire range of temperature and concentrations, indicating the presence of weak interaction between [hmim][FAP] and MDEA. Excess refractive index values showed negative trend over the whole compositions and temperatures.
References
Han X, Armstrong DW. Ionic liquids in separations. Acc Chem Res. 2007;40:1079–86.
Navarro P, Larriba M, Beigbeder JB, Garcia J, Rodriguez F. Thermal stability and specific heats of [bpy][BF4] + [bpy][Tf2 N] and [bpy][BF4] + [4bmpy][Tf2 N] mixed ionic liquid solvents. J Therm Anal Calorim. 2015;119:1235–43.
Chen Y, Zhang S, Yuan X, Zhang Y, Zhang X, Dai W, Mori R. Solubility of CO2 in imidazolium-based tetrafluoroborate ionic liquids. Thermochim Acta. 2006;441:42–4.
Usula M, Plechkova NV, Piras A, Porcedda S. Ethylammonium alkanoate-based ionic liquid + water mixtures. A calorimetric and volumetric study at 298.15 K. J Therm Anal Calorim. 2015;. doi:10.1007/s10973-015-4753-3.
Ziyada AK, Bustam MA, Wilfred CD, Murugesan T. Densities, viscosities, and refractive indices of 1-hexyl-3-propanenitrile Imidazolium ionic liquids incorporated with sulfonate-based anions. J Chem Eng Data. 2011;56:2343–8.
Bukalak D, Kuceba IM, Nowak W. Assessment of the sorption capacity and regeneration of carbon dioxide sorbents using thermogravimetric method. J Therm Anal Calorim. 2013;113:157–60.
Cadena C, Anthony JL, Shah JK, Morrow TI, Brennecke JF, Maginn EJ. Why is CO2 So soluble in imidazolium-based ionic liquids? J Am Chem Soc. 2004;126:5300–8.
Aki SNVK, Mellein BR, Saurer EM, Brennecke JF. High-pressure phase behavior of carbon dioxide with imidazolium-based ionic liquids. J Phys Chem B. 2004;108:20355–65.
Yuan X, Zhang S, Liu J, Lu X. Solubilities of CO2 in hydroxyl ammonium ionic liquids at elevated pressures. Fluid Phase Equilib. 2007;257:195–200.
Tang J, Sun W, Tang H, Radosz M, Shen Y. Enhanced CO2 absorption of poly(ionic liquid)s. Macromolecules. 2005;38:2037–9.
Ahmady A, Hashim MA, Aroua MK. Absorption of carbon dioxide in the aqueous mixtures of methyldiethanolamine with three types of imidazolium-based ionic liquids. Fluid Phase Equilib. 2011;309:76–82.
Sairi NA, Yusoff R, Alias Y, Aroua MK. Solubilities of CO2 in aqueous N-methyldiethanolamine and guanidinium trifluoromethanesulfonate ionic liquid systems at elevated pressures. Fluid Phase Equilib. 2011;300:89–94.
Camper D, Bara JE, Gin DL, Noble RD. Room-temperature ionic liquid—amine solutions: tunable solvents for efficient and reversible capture of CO2. Ind Eng Chem Res. 2008;47:8496–8.
Feng Z, Gang FC, Ting WY, Tao WY, Min LA, Bing ZZ. Absorption of CO2 in the aqueous solutions of functionalized ionic liquids and MDEA. Chem Eng J. 2010;160:691–7.
Rho SW, Yoo KP, Lee JS, Nam SC, Son JE, Min BM. Solubility of CO2 in aqueous methyldiethanolamine solutions. J Chem Eng Data. 1997;42:1161–4.
Taib MM, Murugesan T. Densities and excess molar volumes of binary mixtures of bis(2-hydroxyethyl)ammonium acetate + water and monoethanolamine + Bis(2-hydroxyethyl)ammonium acetate at temperatures from (303.15 to 353.15) K. J Chem Eng Data. 2010;55:5910–3.
Taib MM, Ziyada AK, Wilfred CD, Murugesan T. Thermophysical properties of 1-propyronitrile-3-hexylimidazolium bromide + methanol at temperatures (293.15 to 323.15) K. J Mol Liq. 2011;158:101–4.
Akbar MM, Murugesan T. Thermophysical properties of 1-hexyl-3-methylimidazolium tetrafluoroborate [hmim][BF4] + N-methyldiethanolamine (MDEA) at temperatures (303.15 to 323.15) K. J Mol Liq. 2013;177:54–9.
Alghawas HA, Hagewiesche DP, Ibanez GR, Sandall OC. Physiochemical properties important for carbon dioxide absorption in aqueous methyldiethanolamine. J Chem Eng Data. 1989;34:385–91.
Garcia JMB, Estrada MR, Silva GAI, Hall KR. Densities and excess molar volumes of aqueous solutions of n-methyldiethanolamine (MDEA) at temperatures from (283.15 to 363.15) K. J Chem Eng Data. 2003;48:1442–5.
Alvarez E, Cerdeira F, Gomez-Diaz D, Navaza JM. Density, speed of sound, isentropic compressibility, and excess volume of (monoethanolamine + 2-amino-2-methyl-1-propanol), (monoethanolamine + triethanolamine), and (monoethanolamine + N-methyldiethanolamine) at temperatures from (293.15 to 323.15) K. J Chem Eng Data. 2010;55:994–9.
Muhammad A, Mutalib MIA, Wilfred CD, Murugesan T, Shafeeq A. Viscosity, refractive index, surface tension, and thermal decomposition of aqueous N-Methyldiethanolamine solutions from (298.15 to 338.15) K. J Chem Eng Data. 2008;53:2226–9.
Souckova M, Klomfar J, Patek J. Temperature dependence of the surface tension and 0.1 MPa density for 1-C n -3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate with n = 2, 4, and 6. J Chem Thermodyn. 2012;48:267–75.
Kondaiah M, Sreekanth K, Kumar DS, Nayeem SM, Rao DK. Densities, viscosities, and excess properties for binary mixtures of ethylene glycol with amides at 308.15 K. J Therm Anal Calorim. 2014;118:475–83.
Sankar MG, Ponneri V, Kumar KS, Sakamuri S. Molecular interactions between amine and cyclic ketones at different temperatures. J Therm Anal Calorim. 1821;115:1821–7.
Vural US, Muradoglu V, Vural S. Excess molar volumes, and refractive index of binary mixtures of glycerol + methanol and glycerol + water at 198.15 K and 303.15 K. Bull Chem Soc Ethiop. 2011;25(1):111–8.
Sudake Y, Kamble SP, Tidar AL, Maharolkar AP, Patil SS, Khirade PW. Study of intermolecular interaction of Allyl chloride with 2-pentanone and 2-hexanone through excess molar volume and excess molar refraction. J Pharm Biol Chem Sci. 2011;2:761–70.
Akbar MM, Murugesan T. Thermophysical properties for the binary mixtures of 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide [hmim][Tf2N] + N-methyldiethanolamine (MDEA) at temperatures (303.15 to 323.15)K. J Mol Liq. 2012;169(169):95–101.
Sovilj M, Barjaktarovic B. Excess molar volumes of ternary liquid systems containing aliphatic alcohols at several temperatures. Bull Chem Technol Maced. 2000;19:73–8.
Reis JCR, Lampreia IMS, Santos AFS, Moita MLCJ, Douheret G. Refractive index of liquid mixtures: theory and experiment. ChemPhysChem. 2010;11:3722–33.
Acknowledgements
The financial support by MyRA-Malaysia incentive grant through CO2 Rich Natural Gas Value Chain Program is highly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akbar, M.M., Chemat, F., Arunagiri, A. et al. Density and excess properties of N-methyldiethanolamine (MDEA) with 1-hexyl-3-methylimidazolium tris(pentafluoroethyl)trifluorophosphate [hmim][FAP]. J Therm Anal Calorim 123, 785–791 (2016). https://doi.org/10.1007/s10973-015-4957-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4957-6