Abstract
In recent years, studies on mixtures consisting of ionic liquids and organic solvents have gained importance for the application of such mixtures for new chemical processes and technologies in industries. In this contribution, new experimental excess molar enthalpies, \( H_{\text{ijk}}^{\text{E}} \) data of ternary 1-butyl-2,3-dimethylimidazolium tetrafluoroborate, [Bmmim][BF4] (i) + 1-butyl-3-methylimidazolium tetrafluoroborate, [Bmim][BF4] or 1-ethyl-3-methylimidazolium tetrafluoroborate, [Emim][BF4] (j) + cyclopentanone (CPO) or cyclohexanone (CHO) (k) mixtures, have been reported over the whole composition range at 298.15 K and atmospheric pressure. The observed data have been satisfactorily correlated by Redlich–Kister equation for each mixture. The \( H_{\text{ijk}}^{\text{E}} \) values for [Bmmim][BF4] (i) + [Bmim][BF4] or [Emim][BF4] (j) + CPO (k) mixtures are positive over whole range of composition of xi and xj. The sign and magnitude of \( H_{\text{ijk}}^{\text{E}} \) values for [Bmmim][BF4] (i) + [Bmim][BF4] or [Emim][BF4] (j) + CHO (k) mixtures vary with the change in composition of the components of the mixtures. The \( H_{\text{ijk}}^{\text{E}} \) data have also been analyzed in terms of graph theory (which involves the topology of the molecule). It has been observed that estimated values by graph theory compare well with their corresponding experimental values.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ionic liquids (ILs) generally exhibit peculiar properties [1,2,3,4] such as broad electrochemical window, negligible vapor pressure, low melting points, low toxicity, nonflammability, high ionic conductivity, air- and water-stable behavior, wide liquid range, excellent solubility, non-volatility, recyclability, reusability, high thermal and electrochemical stability and good selectivity which are considered to be excellent fluids for use in a wide range of engineering and material applications such as chemical reactions [5], chemical extractive processes [6], electrochemistry [7], multiphase bioprocess operations [8], liquid–liquid separations [9], batteries and fuel cells [10], synthesis [11], heat transfer fluids in solar heating and absorption refrigerating systems [12]. However, a number of engineering parameters need to be determined for the ILs or their mixtures with organic solvents in order to access their applicability to material applications and process design. Thermodynamic properties of liquid mixtures are required for the various heat flow, mass transfer, heat transfer calculations, and for designing, optimization and simulation of various industrial processes [13,14,15,16]. In particular, excess molar enthalpies, HE, data are essential for the design of chemical reactors and heat transfer systems, which involve mixture/s comprised of ILs or their mixture with organic solvents.
ILs: 1-butyl-2,3-dimethylimidazolium tetrafluoroborate, [Bmmim][BF4], 1-butyl-3-methylimidazolium tetrafluoroborate, [Bmim][BF4], 1-ethyl-3-methylimidazolium tetrafluoroborate, [Emim][BF4], have shown a substantial potential for many technological applications improving operational safety in many systematic processes [17], in bioreactor technology and in nanotechnology [18, 19]. Cyclic ketones are further important intermediates in the synthesis of many organic compounds such as alkoxides, phosphine oxides, used to create fragrances, in chewing gum, in polymers, cosmetic and pharmaceutical industries [20,21,22,23]. A literature survey has revealed that HE values of liquid mixtures containing ILs and organic solvents can be used in variety of applications such as extractive desulphurization of liquid fuels and electrochemical capacitors [24,25,26].
In recent studies [27, 28], we have measured excess properties (excess molar volumes, VE, excess isentropic compressibilities, \( \kappa_{\text{S}}^{\text{E}} \), excess molar enthalpies, HE, and excess heat capacities (\( C_{\text{P}}^{\text{E}} \)) of the binary mixtures containing ILs: [Bmmim][BF4], [Bmim][BF4], [Emim][BF4] and cycloalkanone. The measured data have been successfully analyzed in terms of graph theory which in turn deals with the topology of a molecule. This paper continues our investigations on mixtures comprised of [Bmmim][BF4] or [Bmim][BF4] or [Emim][BF4] or cycloalkanone and reports excess molar enthalpies, \( H_{\text{ijk}}^{\text{E}} \) data of ternary [Bmmim][BF4] (i) + [Bmim][BF4] or [Emim][BF4] (j) + cyclopentanone (CPO) or cyclohexanone (CHO) (k) mixtures over the entire composition range at 298.15 K. Such \( H_{\text{ijk}}^{\text{E}} \) data may thus be of importance to meet the academic and industrial development demands of the society.
Experimental
The ILs, CPO and CHO of highest purity (commercially available) were used in the present investigation. ILs studied in this paper were 1-butyl-2,3-dimethylimidazolium tetrafluoroborate [Bmmim][BF4]; (mass fraction, w: 0.990), 1-butyl-3-methylimidazolium tetrafluoroborate [Bmim][BF4]; (w: 0.985) and 1-ethyl-3-methylimidazolium tetrafluoroborate [Emim][BF4]; (w: 0.990) obtained from Sigma-Aldrich. ILs were purified by vacuum treatment at residual pressure 5 × 10−2 Pa and at 338 K to eliminate the water and other volatile compound traces. The CPO (w: 0.990) and CHO (w: 0.990) were purified by standard means [29], and their final purity was checked by gas chromatography. The w of water in ILs and organic solvents was tested regularly by Karl Fischer titration method [30] and was observed to have maximum value of 0.0003. The purity, supplier, CAS number and analysis methods of the studied chemicals are reported in Table 1. The densities, ρ, and speeds of sound, u, values of the present ILs and cycloalkanones were measured, at 298.15 K and atmospheric pressure using a density and sound analyzer (Anton Paar DSA 5000) with an estimated accuracy of ± 1.2 kg m−3 and ± 0.5 m s−1, respectively, in the manner as described elsewhere [31, 32]. The working frequency of the instrument was 3 MHz. Such ρ and u values are reported and compared with their literature values [25, 26, 33,34,35,36,37,38,39,40,41,42,43,44] in Table 2.
The \( H_{\text{ijk}}^{\text{E}} \) data of the mixtures were measured by means of high-sensitivity micro-differential scanning calorimeter Micro DSC (Model–μDSC 7 Evo), supplied M/S SETARAM, France, in the manner as described elsewhere [45]. The calorimeter uses a double-stage temperature control with Peltier coolers, and the minimum and maximum temperatures that can be reached are about (228.15–393.15) K, respectively. The temperature of the calorimeter was maintained at 298.15 K with the uncertainty of ± 0.02 K. A constant sweeping of nitrogen gas for about four hours (0.3–0.4) MPa pressure was supplied to avoid steam condensation in the calorimeter walls. After this period, 0.08 MPa pressure of nitrogen gas was maintained. The calibration of calorimeter was done by Joule effect method. The calibration was checked by measuring heat of fusion of naphthalene, which was found to be 148.41 J g−1 which in turn was comparable to literature value of 148.7 J g−1 [46]. The \( H_{\text{ijk}}^{\text{E}} \) values for (i + j + k) mixtures were measured by taking binary mixture of known composition (by mass) in the lower chamber of mixing batch cell and pure component (k) (by mass) in the upper chamber of mixing batch cell. The liquid in the upper vessel of mixing batch cell was taken with the help of micropipette (supplied by M/S SETARAM, capacity 10–50 μl). The composition of liquid mixtures was prepared by mass using a digital electronic balance (Mettler AX-205) with an uncertainty of ± 1 × 10−5 g. The uncertainty in the estimation of mole fraction is ± 1 × 10−3. The stability in the calorimeter signal was indicated by consistent heat flow and temperature line on the experimental setup screen. The temperature and isothermal levels were maintained by software provide by M/S SETARAM. After attaining the stability, the knob of the upper chamber of the mixing batch cell was pressed to inject component (k) to the lower chamber possessing (i + j) mixture. After isothermal level time, the data were automatically transferred to the experimental result tab and a graph was obtained between heat flow versus time. The area of the peak provided the amount of heat involved, Qijk, during the mixing process. The Qijk values were then used to determine \( H_{\text{ijk}}^{\text{E}} \) using
where Qij is the amount of heat evolved in the binary (i + j) mixtures of known composition and is calculated using \( H_{\text{ij}}^{\text{E}} \) values of (i + j) mixtures by using
where ni, nj and nk are number of moles of components in ternary mixtures. The \( H_{ij}^{\text{E}} \) values of binary (i + j), (j + k), (i + k) mixtures constituting ternary mixtures were taken from the literature [27, 28, 40, 47]. The uncertainty in the determination of \( H_{\text{ijk}}^{\text{E}} \) values is ± 1%.
Results
Table 3 presents the measured excess molar enthalpies, \( H_{\text{ijk}}^{\text{E}} \) values for ternary [Bmmim][BF4] (i) + [Bmim][BF4] or [Emim][BF4] (j) + CPO or CHO (k) mixtures over the entire mole fraction of (i) and (j) components at 298.15 K. The experimental \( H_{\text{ijk}}^{\text{E}} \) values for each mixture were correlated by means of Redlich–Kister equation [48] in the form:
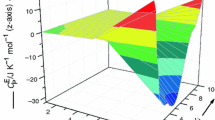
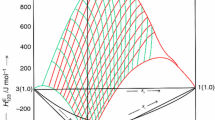
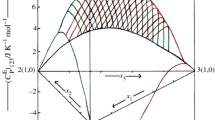
where, xi, xj and xk are the mole fractions of (i), (j) and (k) components. The Redlich–Kister parameters, aij(n), ajk(n), aik(n), (n = 0–2) of binary mixtures (i + j), (j + k), (i + k) of (i + j + k) ternary mixture, were taken from the literature [27, 28, 40, 47] and are also reported in Table 4. The HE data for (i + j), (j + k), (i + k) of (i + j + k) ternary mixture have been measured in the same laboratory by using same chemicals of comparable purity. The aijk(n) (n = 0–2) are coefficients of (i + j + k) mixture and are obtained by fitting the measured \( H_{\text{ijk}}^{\text{E}} \) data to Eq. (3) by least-squares methods. These coefficients along with the standard deviations, \( \sigma \left( {H_{\text{ijk}}^{\text{E}} } \right) \), are presented in Table 5. The surfaces generated by \( H_{\text{ijk}}^{\text{E}} \) values for the studied ternary mixtures are shown in Figs. 1–4. In Fig. 1, the \( H_{\text{ijk}}^{\text{E}} \) values (corresponding to i–j axis) were obtained by keeping xk constant and varying the values of xi and xj (shown as red line); \( H_{\text{ijk}}^{\text{E}} \) values (corresponding to j–k axis) were obtained by keeping xi constant and varying the values xj and xk (shown as green line).
Excess molar enthalpies, \( H_{\text{ijk}}^{\text{E}} \) for 1-butyl-2,3-dimethylimidazolium tetrafluoroborate (i) + 1-butyl-3-methylimidazolium tetrafluoroborate (j) + cyclopentanone (k) mixture at 298.15 K; experimental data in front of the plane (solid line); experimental data behind the plane (dashed line)
Excess molar enthalpies, \( H_{\text{ijk}}^{\text{E}} \) for 1-butyl-2,3-dimethylimidazolium tetrafluoroborate (i) + 1-butyl-3-methylimidazolium tetrafluoroborate (j) + cyclohexanone (k) mixture at 298.15 K; experimental data in front of the plane (solid line); experimental data behind the plane (dashed line)
Excess molar enthalpies, \( H_{\text{ijk}}^{\text{E}} \) for 1-butyl-2,3-dimethylimidazolium tetrafluoroborate (i) + 1-ethyl-3-methylimidazolium tetrafluoroborate (j) + cyclopentanone (k) mixture at 298.15 K; experimental data in front of the plane (solid line); experimental data behind the plane (dashed line)
Excess molar enthalpies, \( H_{\text{ijk}}^{\text{E}} \) for 1-butyl-2,3-dimethylimidazolium tetrafluoroborate (i) + 1-ethyl-3-methylimidazolium tetrafluoroborate (j) + cyclohexanone (k) mixture at 298.15 K; experimental data in front of the plane (solid line); experimental data behind the plane (dashed line)
Discussion
We are unaware of any published \( H_{\text{ijk}}^{\text{E}} \) data of investigated mixtures with which to compare our results. The \( H_{\text{ijk}}^{\text{E}} \) values for [Bmmim][BF4] (i) + [Bmim][BF4] or [Emim][BF4] (j) + CPO (k) mixtures are endothermic over whole range of composition of xi and xj. However, sign and magnitude of \( H_{\text{ijk}}^{\text{E}} \) values for [Bmmim][BF4] (i) + [Bmim][BF4] or [Emim][BF4] (j) + CHO (k) mixtures are dictated by relative proportion of the components in the mixture. The \( H_{\text{ijk}}^{\text{E}} \) results from the disruption of interactions among the like molecules and the introduction of new interactions between the unlike molecules. The endothermic behavior of [Bmmim][BF4] (i) + [Bmim][BF4] or [Emim][BF4] (j) + CPO (k) mixtures reveals that the contribution to \( H_{\text{ijk}}^{\text{E}} \) due to disruption of cohesion forces in ILs and dipole–dipole interaction in CPO far outweighs the contribution due to interaction of CPO with [Bmmim][BF4]:[Bmim][BF4] and [Bmmim][BF4]:[Emim][BF4] molecular entities. The \( H_{\text{ijk}}^{\text{E}} \) values for [Bmmim][BF4] (i) + [Emim][BF4] (j) + CHO (k) are lesser than [Bmmim][BF4] (i) + [Emim][BF4] (j) + CPO (k) mixture which in turn indicate strong interactions among the CHO and [Bmmim][BF4]:[Emim][BF4] in comparison with CPO and [Bmmim][BF4]:[Emim][BF4] molecular entity. This may be due to behavior of CHO (being more basic in nature than CPO and also possess chair form with almost no strain) that results in strong interactions/effectively packing of CHO in the [Bmmim][BF4]:[Emim][BF4] framework as compared to CPO. Further, the higher \( H_{\text{ijk}}^{\text{E}} \) values for [Bmmim][BF4] (i) + [Bmim][BF4] (j) + CPO or CHO (k) than [Bmmim][BF4] (i) + [Emim][BF4] (j) + CPO or CHO (k) mixtures suggest least interactions between CPO or CHO with [Bmmim][BF4]:[Bmim][BF4] in comparison with [Bmmim][BF4]:[Emim][BF4] molecular entity. This trend may be explained on the basis of steric effect where the presence of bulky butyl group in side chain of [Bmim][BF4] obstructs the approach of CPO or CHO toward [Bmmim][BF4]:[Bmim][BF4] in comparison with [Bmmim][BF4]:[Emim][BF4] molecular entity.
Graph theory
Conceptual aspects of graph theory
Molecular topology has been widely used in a variety of areas such as in the discovery and design of new drugs, molecule design, prediction of physicochemical parameters, pharmacological properties, mathematical models for the selection and design of new active compounds [49]. Molecular topology of a molecule depends upon the manner in which the components of a particular species are being associated with each other and thus correlates between a given physical, chemical, or biological property with the corresponding molecular characterization provided by some numerical invariants known as topological indices. The purpose of these indices is to codify the physicochemical properties of a molecule in a purely numerical fashion. A variety of topological indices [50,51,52,53] have been proposed, and a number of investigations have been made to extend and apply them in structure activity studies for encoding the structural information. The connectivity parameter of third degree of a molecule, \( ^{3} \xi \) (which in turn depends upon its topology), has been successfully utilized to build relation between the thermodynamic properties (VE, \( \kappa_{\text{S}}^{\text{E}} \), HE, \( C_{\text{P}}^{\text{E}} \)) of binary/ternary liquid mixtures and the corresponding molecular characterization provided by the constituents of mixtures. In the present study, connectivity parameters of third degree of the constituent molecules of mixtures have been successfully utilized to predict \( H_{ijk}^{\text{E}} \) data of ternary mixtures.
Graph theory and results
The HE data of liquid mixtures can be tested in terms of graph theory if the states of components in pure and mixed states are known. In our earlier studies [27, 28, 40, 47], thermodynamic and topological analyses of excess properties (VE, \( \kappa_{\text{S}}^{\text{E}} \), HE and \( C_{\text{P}}^{\text{E}} \)) of [Bmmim][BF4] (i) + [Bmim][BF4] or [Emim][BF4] (j); [Bmmim][BF4] or [Bmim][BF4] or [Emim][BF4] (i) + CPO or CHO (j) binary mixtures have suggested that ILs: [Bmmim][BF4], [Bmim][BF4] and [Emim][BF4], are characterized by cohesion forces between (a) hydrogen atom of C–H (edge) of imidazolium ring and two fluorine atoms of BF4; (b) proton of CH3 group of imidazolium ring and two fluorine atoms of BF4 and exists as monomer; (c) CPO or CHO is characterized by dipole–dipole interactions and exists as associated molecular entities.
Quantum mechanical and infrared (IR) spectral studies also support this view point [27, 28, 40, 47, 54,55,56,57,58,59,60,61].
Scheme 1 represents the connectivity parameters of third degree of the components [27, 28, 40, 47] and various inter-nuclear distances among interacting atoms (predicted by quantum mechanical calculations using Gaussian program package 09) in [Bmmim][BF4], [Bmim][BF4], [Emim][BF4], CPO and CHO.
The energetics of ternary mixtures can be studied if it be assumed that the addition of component (k) to (i + j) mixture leads to the formation of (i + j + k) mixture that may involve the processes: (I) formation of unlike (a) i − j, (b) j − kn (n = 2), (c) i − kn contacts and (II) unlike contact formation then cause the rupture of (a–b) cohesion forces in pure ILs, [Bmmim][BF4] or [Bmim][BF4] or [Emim][BF4], and (c) dipole–dipole interactions in CPO or CHO to yield their respective monomers; (III) specific interactions between i, j and k molecules lead to the formation of (a) i:j; (b) j:k; and (c) i:k molecular complexes.
If \( \chi_{\text{ij}} ,\chi_{\text{jk}} ;\;\chi_{\text{ii}} ,\chi_{\text{jj}} \chi_{\text{kk}} \)\( \chi_{\text{ik}} \); and \( \chi_{\text{ij}}^{/} ,\chi_{\text{jk}}^{//} \chi_{\text{ik}}^{///} \) are molar interaction parameters for (I) establishment of unlike (a) i − j, (b) j − kn (n = 2), (c) i − kn contacts; (II) rupture of cohesion forces (a–b) in ILs and (c) dipole–dipole interactions in CPO and CHO; and (III) formation of i:j, j:k, i:k molecular complexes, respectively.
Then change in molar enthalpies, ∆H, due to processes (I) (a)–(c); (II) (a)–(c); and (III) (a)–(c) are then expressed by [62,63,64]:
where vi, vj and vk are the molar volumes of components (i), (j) and (k), respectively.
The total change in \( H_{\text{ijk}}^{\text{E}} \) values due to processes: (I) (a)–(c); (II) (a)–(c); and (III) (a)–(c) is presented by:
It has further been shown [65] that \( 1/^{3} \xi \) of a molecule represents a measure of the probability that its surface area interacts effectively with the corresponding surface area of other molecule and within the same isomeric species; molar volume of a molecule varies inversely as its \( ^{3} \xi \). Consequently, \( {{v_{\text{j}} } \mathord{\left/ {\vphantom {{v_{\text{j}} } {v_{\text{i}} }}} \right. \kern-0pt} {v_{\text{i}} }} = \left( {{}^{3}\xi_{\text{i}} /{}^{3}\xi_{\text{j}} } \right) \) [66] where \( \left( {{}^{3}\xi_{\text{i}} } \right) \), (i = i or j or k) are the connectivity parameter of third degree of components (i), (j) and (k), respectively, and are defined by
where \( \delta_{\text{m}}^{\varvec{v}} \) values define [67] the valency of mth vertex used in the formation of the bond and has been calculated by employing relation \( \delta^{v} = Z_{\text{m}} {-}h \). (Zm is related to maximum valency of atom and h is the number of hydrogen atoms attached to it.)
Equation 7 is, therefore, reduced to:
In the present mixtures, it was assumed that (1) molar interaction parameters for the formation of unlike contacts i − j; j − kn; i − kn are nearly equal to molar interaction parameters for the establishment of i:j, j:k, i:k molecular complexes, and (2) molar interaction parameter for the dissociation of cohesion forces in ILs and dipole–dipole interactions in cycloalkanone is nearly equal, i.e., \( \chi_{\text{ij}} \cong \chi_{\text{ij}}^{/} = \chi_{\text{ij}}^{ * } ;\;\chi_{\text{jk}} \cong \chi_{\text{jk}}^{//} = \chi_{\text{jk}}^{ * } ;\;\chi_{\text{ik}} \cong \chi_{\text{ik}}^{///} = \chi_{\text{ik}}^{ * } ;\;\chi_{\text{ii}} \cong \chi_{{{\text{jj}}\,}} \cong \chi_{\text{kk}} = \chi^{*} \); Eq. (9) is then expressed as:
The four unknown parameters: \( \chi_{\text{ij}}^{ * } \), \( \chi_{\text{jk}}^{ * } \), \( \chi_{\text{ik}}^{ * } \) and \( \chi^{ * } \), in Eq. (10) are commuted using \( H_{\text{ijk}}^{\text{E}} \) data at four arbitrary compositions and then subsequently used to calculate \( H_{\text{ijk}}^{\text{E}} \) values at other mole fractions of xi and xj. Such \( H_{\text{ijk}}^{\text{E}} \) values for investigated mixtures are reported in Table 3 and also compared with the corresponding experimental values. The \( \chi_{\text{ij}}^{ * } \), \( \chi_{\text{jk}}^{ * } \), \( \chi_{\text{ik}}^{ * } \) and \( \chi^{ * } \) parameters are listed in Table 6 along with standard deviations, σ(\( H_{\text{ijk}}^{\text{E}} \)) between experimental and theoretical calculated values. A perusal of data in Table 3 indicates a good agreement between calculated (by graph theory) and experimental \( H_{\text{ijk}}^{\text{E}} \) data, which in turn support the various assumptions made regarding the various processes involved in the mixture formation and also in deriving Eq. (10).
Conclusions
The present work reports excess molar enthalpies, \( H_{\text{ijk}}^{\text{E}} \) data for [Bmmim][BF4] (i) + [Bmim][BF4] or [Emim][BF4] (j) + CPO or CHO (k) mixtures over the entire mole fraction of (i) and (j) components at 298.15 K. The analyses of \( H_{\text{ijk}}^{\text{E}} \) data of [Bmmim][BF4] (i) + [Emim][BF4] (j) + CHO (k) mixture suggest that CHO molecules have been effectively packed in the lattices of [Bmmim][BF4]:[Emim][BF4] molecular entity in comparison with CPO molecules. However, reverse is the trend in case of [Bmmim][BF4] (i) + [Bmim][BF4] (j) + CHO (k) mixture. The \( H_{\text{ijk}}^{\text{E}} \) values of studied (i + j + k) mixtures also indicate least interactions between CPO and CHO with [Bmmim][BF4]:[Bmim][BF4] in comparison with [Bmmim][BF4]:[Emim][BF4]. The \( H_{\text{ijk}}^{\text{E}} \) data of the present mixtures have also been tested in terms of graph theory. It has been observed that \( H_{\text{ijk}}^{\text{E}} \) values computed by graph theory are in agreement with experimental values.
References
Nebig S, Bölts R, Gmehling J. Measurement of vapor–liquid equilibria (VLE) and excess enthalpies (H E) of binary systems with 1-alkyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide and prediction of these properties and γ using modified UNIFAC (Dortmund). Fluid Phase Equilib. 2007;258:168–78.
Safarov J, Hassel E. Thermodynamic properties of 1-hexyl-3-methylimidazolium tetrafluoroborate. J Mol Liq. 2010;153:153–8.
Shamsipur M, Miran Beigi AA, Teymouri M, Pourmortazavi SM, Irandoust M. Physical and electrochemical properties of ionic liquids 1-ethyl-3- methylimidazolium tetrafluoroborate, 1-butyl-3-methylimidazolium trifluoromethanesulfonate and 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide. J Mol Liq. 2010;157:43–50.
Gaciño FM, Paredes X, Comuñas MJP, Fernández J. Pressure dependence on the viscosities of 1-butyl-2,3-dimethylimidazolium bis(trifluoromethylsulfonyl)imide and two tris(pentafluoroethyl)trifluorophosphate based ionic liquids: new measurements and modelling. J Chem Thermodyn. 2013;62:162–9.
Fan XH, Chen YP, Su CS. Density and Viscosity Measurements for binary mixtures of 1-ethyl-3-methylimidazolium tetrafluoroborate ([Emim][BF4]) with dimethylacetamide, dimethylformamide, and dimethyl sulfoxide. J Chem Eng Data. 2016;61:920–7.
Shekaari H, Kazempour A. Solution properties of ternary d-glucose + 1-ethyl-3- methylimidazolium ethyl sulfate + water solutions at 298.15 K. J Solut Chem. 2011;40:1582–95.
Yang JZ, Guan W, Tong J, Wang H, Li L. Studies of thermochemical properties of a new ionic liquid prepared by mixing 1-methyl-3-pentylimidazolium chloride with InCl3. J Solut Chem. 2006;35:845–52.
Altuwaim MS, Alkhaldi KHAE, Al-Jimaz AS, Mohammad AA. Temperature dependence of physicochemical properties of imidazolium-, pyroldinium-, and phosphonium-based ionic liquids. J Chem Eng Data. 2014;59:1955–63.
Dai Y, Qu Y, Wang S, Wang J. Measurement, correlation, and prediction of vapor pressure for binary and ternary systems containing an alkylsulfate-based ionic liquid. Fluid Phase Equilib. 2015;397:58–67.
Chun Hua H, Sorianoa AN, Lerona RB, Hui Li M. Molar heat capacity of four aqueous ionic liquid mixtures. Thermochim Acta. 2011;519:44–9.
Shekaari H, Zafarani-Moattar MT, Mirheydari SN. Density, viscosity, speed of sound, and refractive index of a ternary solution of aspirin, 1-butyl-3-methylimidazolium bromide, and acetonitrile at different temperatures T = (288.15 to 318.15) K. J Chem Eng Data. 2015;60:1572–83.
Kim KI, Shin BK, Ziegler F. XV international symposium of thermophysical properties, Colorado, U.S.A; 2003.
Raeissia S, Petersb CJ. Density, viscosity, speed of sound, and refractive index of a ternary solution of aspirin, 1-butyl-3-methylimidazolium bromide, and acetonitrile at different temperatures T = (288.15 to 318.15) K. Fluid Phase Equilib. 2010;294:67–71.
Choraüzewski M, Tkaczyk M. Heat capacity, speed of ultrasound, and density for 1,5-dibromopentane + heptane within the temperature range from 293.15 K to 313.15 K. J Chem Eng Data. 2006;51:1825–31.
Oliveira MB, Domínguez-Pérez M, Cabeza O, Lopes-da-Silva JA, Freire MG, Coutinho JAP. Surface tensions of binary mixtures of ionic liquids with bis(trifluoromethylsulfonyl)imide as the common anion. J Chem Thermodyn. 2013;64:22–7.
Gnanakumari P, Venkatesu P, Rama Mohan K, Prabhakara Rao MV, Prasad DHL. Excess volumes and excess enthalpies of N-methyl-2-pyrrolidone with branched alcohols. Fluid Phase Equilib. 2007;252:137–42.
Palm R, Kurig H, Tonurist K, Janes A, Lust E. Is the mixture of 1-ethyl-3-methylimidazolium tetrafluoroborate and 1-butyl-3-methylimidazolium tetrafluoroborate applicable as electrolyte in electrical double layer capacitors? Electrochem Commun. 2012;22:203–6.
Chaurasia SK, Singh RK, Chandra S. Structural and transport studies on polymeric membranes of PEO containing ionic liquid, EMIM-TY: evidence of complexation. Solid State Ion. 2011;183:32–9.
Gonzalez EJ, Requejo PF, Dominguez A, Macedo EA. Physical properties of binary alcohol + ionic liquid mixtures at several temperatures and atmospheric pressure. J Solut Chem. 2013;42:746–63.
Balaji R, Sankar MG, Sekhar MC, Shekar MC. Thermophysical and spectroscopic properties of binary liquid systems: acetophenone/cyclopentanone/cyclohexanone with N-methylformamide. Phys Chem Liq. 2016;54:422–39.
Dragoescu D. Refractive indices and their related properties for several binary mixtures containing cyclic ketones and chloroalkanes. J Mol Liq. 2015;209:713–22.
Kumari PG, Venkatesu P, Hofman T, Rao MVP. Excess molar enthalpies and vapor–liquid equilibrium for N-methyl-2-pyrrolidone with ketones. J Chem Eng Data. 2010;55:69–73.
Gowrisankar M, Venkateswarlu P, Siva Kumar K, Sivarambabu S. Volumetric, speed of sound data and viscosity at (303.15 and 308.15) K for the binary mixtures of N,N-dimethylaniline + aliphatic ketones (C3–C5), +4-methyl-2-pentanone, +acetophenone, +cyclicketones. J Ind Eng Chem. 2014;20:405–18.
Andriyko YO, Reischl W, Nauer GE. Trialkyl-substituted imidazolium-based ionic liquids for electrochemical applications: basic physicochemical properties. J Chem Eng Data. 2009;54:855–60.
Ciocirlan O, Iulian O. Properties of pure 1-butyl-2,3-dimethylimidazolium tetrafluoroborate ionic liquid and its binary mixtures with dimethyl sulfoxide and acetonitrile. J Chem Eng Data. 2012;57:3142–8.
Sunkara GR, Tadavarthi MM, Tadekoru VK, Tadikonda SK, Bezawada SR. Density, refractive index, and speed of sound of the binary mixture of 1-butyl-3-methylimidazolium tetrafluoroborate +N-Vinyl-2-pyrrolidinone from T = (298.15 to 323.15) K at atmospheric pressure. J Chem Eng Data. 2015;60:886–94.
Gupta H, Kataria J, Sharma D, Sharma VK. Topological investigations of molecular interactions in binary ionic liquid mixtures with a common ion: excess molar volumes, excess isentropic compressibilities, excess molar enthalpies and excess molar heat capacities. J Chem Thermodyn. 2016;103:189–205.
Gupta H, Solanki S, Sharma VK. Topological analysis of thermodynamic properties of binary mixtures containing 1-butyl-3-methylimidazolium tetrafluoroborate and cycloalkanones. J Therm Anal Calorim. 2017;127:2459–72.
Riddick JA, Bunger WB, Sakano TK. Organic solvents physical properties and methods of purification. 4th ed. New York: Wiley; 1986.
Scholz E. Karl Fischer titration. Berlin: Springer; 1984.
Dubey GP, Sharma M. Temperature and composition dependence of the densities, viscosities, and speeds of sound of binary liquid mixtures of 1-butanol with hexadecane and squalane. J Chem Eng Data. 2008;53:1032–8.
Saini N, Yadav JS, Jangra SK, Sharma D, Sharma VK. Thermodynamic studies of molecular interactions in mixtures of o-toulidine with pyridine and picolines: excess molar volumes, excess molar enthalpies, and excess isentropic compressibilities. J Chem Thermodyn. 2011;43:782–95.
Malham IB, Turmine M. Viscosities and refractive indices of binary mixtures of 1-butyl-3-methylimidazolium tetrafluoroborate and 1-butyl-2,3-dimethylimidazolium tetrafluoroborate with water at 298 K. J Chem Thermodyn. 2008;40:718–23.
Pal A, Kumar B, Kang TS. Effect of structural alteration of ionic liquid on their bulk and molecular level interactions with ethylene glycol. Fluid Phase Equilib. 2013;358:241–9.
Pal A, Kumar B. Volumetric and acoustic properties of binary mixtures of the ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate [bmim][BF4] with alkoxyalkanols at different temperatures. J Chem Eng Data. 2012;57:688–95.
Seki S, Tsuzuki S, Hayamizu K, Umebayashi Y, Serizawa N, Takei K, Miyashiro H. Comprehensive refractive index property for room-temperature ionic liquids. J Chem Eng Data. 2012;57:2211–6.
Stoppa A, Zech O, Kunz W, Buchner R. The conductivity of imidazolium-based ionic liquids from (−35 to 195) °C. A. Variation of Cation’s alkyl chain. J Chem Eng Data. 2010;55:1768–73.
Reddy MS, Nayeem SM, Raju KTSS, Babu BH. The study of solute–solvent interactions in 1-ethyl-3-methylimidazolium tetrafluoroborate +2-ethoxyethanol from density, speed of sound, and refractive index measurements. J Therm Anal Calorim. 2016;124:959–71.
Vercher E, Llopis FJ, Gonzalez-Alfaro V, Miguel PJ, Orchilles V, Martinez- Andreu A. Volumetric properties, viscosities and refractive indices of binary liquid mixtures of tetrafluoroborate-based ionic liquids with methanol at several temperatures. J Chem Thermodyn. 2015;90:174–84.
Sharma VK, Kataria J, Bhagour S. Thermodynamic investigations of 1-ethyl-3-methylimidazolium tetrafluoroborate and cycloalkanone mixtures. J Therm Anal Calorim. 2014;118:431–47.
Ciocirlan O, Teodorescu M, Dragoesce D, Iulian O, Barhala A. Densities and excess molar volumes of the binary mixtures of cyclopentanone with chloroalkanes at T = (288.15, 298.15, 308.15, and 318.15) K. J Chem Eng Data. 2010;55:3891–5.
Dragoescu D, Teodorescu M, Barhala A. Isothermal (vapour + liquid) equilibria and excess Gibbs free energies in some binary (cyclopentanone + chloroalkane) mixtures at temperatures from 298.15 K to 318.15 K. J Chem Thermodyn. 2007;39:1452–7.
Bermudez-Salguero C, Gracia-Fadrique J, Calvo E, Amigo A. Densities, refractive indices, speeds of sound, and surface tensions for dilute aqueous solutions of 2-methyl-1-propanol, cyclopentanone, cyclohexanone, cyclohexanol, and ethyl acetoacetate at 298.15 K. J Chem Eng Data. 2011;56:3823–9.
Ciocirlan O, Teodorescu M, Dragoescu D, Iulian O, Barhala A. Densities and excess molar volumes of the binary mixtures of cyclohexanone with chloroalkanes at temperatures between (288.15 and 318.15) K. J Chem Eng Data. 2010;55:968–73.
Sharma VK, Solanki S, Bhagour S, Sharma D. Excess molar enthalpies of ternary mixtures containing 1-ethyl-3-methylimidazolium tetrafluoroborate and organic solvents. Thermochim Acta. 2013;569:36–41.
Sabbah R, Xu-Wu A, Chickos JS, Leitao MLP, Roux MV, Torres LA. Reference materials for calorimetry and differential thermal analysis. Thermochim Acta. 1999;331:93–204.
Gupta H, Chandrasekhar M, Krishna TS, Sharma VK. Thermodynamic properties of mixtures containing 1-butyl-2,3-dimethylimidazolium tetrafluoroborate and cyclopentanone or cyclohexanone. J Mol Liq. 2017;231:225–37.
Redlich O, Kister AT. Algebraic representation of thermodynamic properties and the classification of solutions. Ind Eng Chem. 1948;40:345–8.
Gupta H, Malik S, Sharma VK. Excess molar volumes and excess isentropic compressibilities of ternary mixtures containing ionic liquids and cyclic alkanone. J Chem Thermodyn. 2017;112:86–102.
Gupta H, Malik S, Chandrasekhar M, Sharma VK. Thermodynamic investigations of excess heat capacities of ternary liquid mixtures containing [Bmmim][BF4] + [Bmim][BF4] or [Emim][BF4] + cyclopentanone or cyclohexanone. J Therm Anal Calorim. 2017. https://doi.org/10.1007/s10973-017-6587-7.
Amigó JM, Gálvez J, Villar VM. A review on molecular topology: applying graph theory to drug discovery and design. Naturwissenschaften. 2009;96:749–61.
Kier LB, Hall LH. Molecular connectivity in chemistry and drug research. New York: Academic Press; 1976.
Hoyosa H. Topological index. A newly proposed quantity characterizing the topological nature of structural isomers of saturated hydrocarbons. Bull Chem Soc Jpn. 1971;44:2332–9.
Balaban AT. Highly discriminating distance-based topological index. Chem Phys Lett. 1982;89:399–404.
Wiener H. Structural determination of paraffin boiling points. J Am Chem Soc. 1947;69:17–20.
Vaz PD, Ribeiro-Claro PJA. Strong experimental evidence of C–H···O hydrogen bonds in cyclopentanone: the splitting of the ν(C=O) mode revisited. J Phys Chem A. 2003;107:6301–5.
Valadbeigi Y, Farrokhpour H, Tabrizchi M. Effect of hydration on the kinetics of proton-bound dimer formation: experimental and theoretical study. J Phys Chem A. 2014;118:7663–71.
Andriyko Y, Andriiko A, Babushkina OB, Nauer GE. Electrochemistry of TiF4 in 1-butyl-2,3-dimethylimidazolium tetrafluoroborate. Electrochim Acta. 2010;55:1081–9.
Rao CNR. Chemical application of infrared spectroscopy. London: Academic Press; 1963.
Swapnil AD, Kailas LW, Mahesh NV, Diwakar ZS, ChangKyoo Y. Synthesis, characterization and application of 1-butyl-3-methylimidazolium tetrafluoroborate for extractive desulfurization of liquid fuel. Arab J Chem. 2016;9:578–87.
Silverstein RM, Bassler GC, Morrik TC. Spectroscopic identification of organic compounds. 5th ed. Singapore: Wiley; 1991.
Huggins ML. The thermodynamic properties of liquids, including solutions. I. Intermolecular energies in monotonic liquids and their mixtures. J Phys Chem. 1970;74:371–8.
Huggins ML. The thermodynamic properties of liquids, including solutions: part 2. Polymer solutions considered as ditonic systems. Polymer. 1971;12:389–99.
Singh PP, Bhatia M. Energetics of molecular interactions in binary mixtures of non-electrolytes containing a salt. J Chem Soc Faraday Trans. 1989;1:3807–12.
Singh PP. Topological aspects of the effect of temperature and pressure on the thermodynamics of binary mixtures of non-electrolytes. Thermochim Acta. 1983;66:37–73.
Singh PP, Nigam RK, Singh KC, Sharma VK. Topological aspects of the thermodynamics of binary mixtures of non-electrolytes. Thermochim Acta. 1981;46:175–90.
Kier LB, Yalkowsky SH, Sinkula AA, Valvani SC. Physico-chemical properties of drugs. New York: Marcel Dekker; 1980. p. 282–95 (Chapter 9).
Acknowledgements
The authors are thankful to Mr. K. Chandrasekhar Reddy, Sri Sai Baba National College, Anantapur, Andhra Pradesh, for providing Gaussian-09 facility and Centre for Development of Advanced Computing (C-DAC), Pune, India, for providing the computational work. V. K. Sharma is grateful to University Grant Commission (UGC), New Delhi, for the award of Special Assistance Programme (SAP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gupta, H., Malik, S. & Sharma, V.K. Excess molar enthalpies for [Bmmim][BF4] + [Bmim][BF4] or [Emim][BF4] + cyclopentanone or cyclohexanone mixtures. J Therm Anal Calorim 136, 1383–1394 (2019). https://doi.org/10.1007/s10973-018-7770-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-7770-1