Abstract
The densities, ρ, and viscosities, η, of binary mixtures of ethylene glycol with formamide, N,N-dimethyl formamide and N,N-dimethyl acetamide, have been measured over the entire composition range at 308.15 K. From this experimental data, excess molar volume, \( V_{\text{m}}^{\text{E}} \), deviation in viscosity, Δη, and excess Gibbs free energy of activation of viscous flow, \( \Delta G^{{ * {\text{E}}}}, \) have been determined. Negative values of \( V_{\text{m}}^{\text{E}} \), Δη, and \( \Delta G^{{ * {\text{E}}}} \) are observed over the entire composition range in the mixtures studied. The observed negative values of various excess and deviation parameters are attributed to the existence of strong interactions, like dipole–dipole interactions, H-bonding between the carbonyl group of amide molecules, and hydroxyl group of glycol molecules, geometrical fitting of smaller molecules into the voids created by larger molecules in the liquid mixtures. The excess properties have been fitted to Redlich–Kister-type polynomial, and the corresponding standard deviations have been calculated. The derived partial molar volumes and excess partial molar volumes also support the \( V_{\text{m}}^{\text{E}} \) results. The experimental viscosity data of all of these liquid mixtures have been correlated with four viscosity models.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The volumetric and viscometric study of liquid mixtures enables the determination of some useful thermodynamic and other properties that are highly sensitive to molecular interactions [1–4]. Binary liquid mixtures containing glycols are used in the pharmaceutical, cosmetic, and food industries [5]. Amides, relevant liquid systems for the study of molecular interactions, are among the most common solvents used in chemical reactions and in many industrial processes, and are important model systems for the investigation of peptide and protein interactions in biological systems. The present work focuses on the study of volumetric and viscometric behavior of binary mixtures of ethylene glycol (EG) with formamide (FA), N,N-dimethyl formamide (DMF), and N,N-dimethyl acetamide (DMA) over the entire composition range at 308.15 K.
In the earlier years, so many researchers carried out the ultrasonic, volumetric, and viscometric studies on binary mixtures, glycol as one component and +2-methoxy ethanol [6], +formamide [7], +2-butoxy ethanol [8], +water [9, 10] as other components. This literature survey shows that the mixing properties of EG+FA/DMF/DMA binary mixtures have not been studied previously. In the present investigation, we report data related to densities, ρ, and viscosities, η, of binary liquid mixtures containing EG with FA/DMF/DMA over the entire composition range at 308.15 K. The experimental data of ρ and η were used to calculate the excess molar volume, \( V_{\text{m}}^{\text{E}} \), deviation in viscosity, Δη, and excess Gibbs free energy of activation of viscous flow, \( \Delta G^{{ * {\text{E}}}} \). The variation of these properties with composition has been discussed in terms of molecular interactions.
Experimental
High purity and analytical reagent (AR) grade compounds of ethylene glycol, formamide, N,N-dimethyl formamide, and N,N-dimethyl acetamide were obtained from LOBA chemicals, India. The above chemicals used in the present investigation were further purified by standard methods [11]. The mass fraction purity of liquids obtained is >0.995. Binary mixtures of EG with FA/DMF/DMA were prepared so that the entire composition range is covered (i.e., 0–100 % of EG). The mixtures were prepared by mass in air-tight bottles. The mass measurements were performed with a METTLER TOLEDO (Switzerland) ABB5-S/FACT digital balance with an accuracy ±0.01 mg. The uncertainty in the mole fraction is 10−4. The density and viscosity measurements of liquid mixtures have been measured using a two-stem double-walled Parker and Parker-type pycnometer [12] and Ostwald viscometer, respectively. The detailed descriptions of measurements of density and viscosity were presented in our previous papers [13–17]. The reproducibilities in the measured parameters of density and viscosity are 3 in 105parts and ±0.2 %, respectively. The units of density and viscosity measurements are kg m−3 and N s m−2, respectively. The experimentally determined values of ρ and η at 308.15 K of all the pure liquids have been compared with the literature data [18–22] in Table 1.
Theory and calculation
The experimental values of density have been used to calculate the molar volume and excess molar volume data with the following equations:
where M 1 and M 2 are the molar masses of the pure components 1 (EG), 2 (FA or DMF or DMA), respectively, and ρ is the density of the mixture.
The deviation/excess parameters are computed with the following equations:
Excess molar volume
Deviation in viscosity
Excess Gibbs free energy of activation for viscous flow
where \( \eta_{1}^{ * } \) and \( V_{1}^{ * } \)are the viscosities and molar volumes of pure component 1 (EG) and \( \eta_{2}^{ * } \) and \( V_{2}^{ * } \) are the viscosities and molar volumes of pure component 2 (FA or DMF or DMA), respectively; and x i represents the mole fraction of the component ‘i’ in the mixture. The reproducibility in the excess molar volumes is twice the reproducibility in the molar volume (reproducibility in the molar volume is 3 in 105 parts).
The detailed calculations of excess/deviation properties have been described in our previous papers [13, 16]. The experimentally measured values of ρ, η, and evaluated values of V m, Δη, and \( \Delta G^{{ * {\text{E}}}} \) for all the systems have been presented in Table 2. The excess molar volumes \( V_{\text{m}}^{\text{E}} \) are presented in Table 3. The values of deviation in viscosity, excess molar volume, and excess Gibbs free energy of activation of viscous flow have been fitted to a Redlich–Kister [23]-type polynomial equation.
where Y E is \( V_{\text{m}}^{\text{E}} \), Δη, and \( \Delta G^{{ * {\text{E}}}} \). The subscript ‘i’ in the summation of the above equation takes values from 0 to 4. The values of coefficients A i in the above equation have been determined using the least square method and are compiled in Table 4 along with the standard deviations σ(Y E) calculated using the expression
where ‘m’ is the total number of experimental points and ‘n’ is the number of coefficients in Eq. (5). The value of ‘n’ in the present study is 5.
Results and discussion
From Table 2, the density is found to decrease in EG+FA system, and it is observed to increase monotonically in EG+DMF/DMA systems, whereas the viscosity is found to increase monotonically and non-linearly in all the binary systems with increase in concentration of EG. This non-linear variation which is a deviation from ideal behavior suggests that interactions exist between molecules of component liquids of the mixtures.
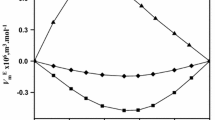
The variations of excess molar volume, \( V_{\text{m}}^{\text{E}}, \) with the mole fraction of EG for the binary systems are shown in Fig. 1. The deviation of physical and chemical properties of the liquid mixture from the ideal behavior is a measure of the interaction between molecules of the components of liquid mixtures, and such a type of deviation is generally attributed to dipole–dipole interactions and hydrogen bond between unlike molecules [24], respectively. The factors that are mainly responsible for the expansion of volume, that is, positive values of \( V_{\text{m}}^{\text{E}} \) are (i) breaking one or both of the components in a solution, that is, loss of dipolar association between the molecules (dispersion forces); (ii) the geometry of molecular structures which does not favor the fitting of molecules of one component into the voids created by the molecules of other component; (iii) steric hindrance of the molecules, where the negative values of \( V_{\text{m}}^{\text{E}} \) are due to strong specific interactions such as (iv) association of molecules through the formation of hydrogen bonds, association due to dipole–dipole interactions, or association due to induced dipole–dipole interactions; and (v) accommodation of molecules due to larger differences in molar volumes. The variation of excess molar volume in the present investigation is negative over the entire mole fraction range. The molar volumes of EG, FA, DMF, and DMA are 56.29, 40.21, 78.12, and 93.93 cm3 mol−1 at 308.15 K, respectively.
EG molecules are self-associated through inter- as well as intra-molecular hydrogen bonding [25], whereas FA molecules are strongly associated through hydrogen bonding due to the presence of strong proton-acceptor group (>C=O) and proton-donor group (–NH2) in their molecules in pure state [1], and this association decreases with increase in the number of methyl groups in the molecule. Thus, DMF and DMA are practically unassociated [26, 27]. FA molecules having hydrogen atoms bonded to nitrogen have both hydrogen-bond donating and hydrogen-bond accepting abilities; on the other hand, DMF and DMA contain only a hydrogen-bond accepting carbonyl group. The addition of amide molecules causes breaking of the hydrogen bonds between EG molecules and the subsequent formation of strong hydrogen bonds between the hydrogen atom of –OH group of glycol molecules and oxygen molecule of >C=O group of amide molecules.
The \( V_{\text{m}}^{\text{E}} \) values are more negative for EG+DMA than that of EG+DMF. This is due to that the electron density at oxygen atom of carbon atom of DMA is greater than that of DMF [28, 29] due to the presence of –CH3 group at the carbon atom of carbonyl group in DMA resulting strong interactions in EG+DMA system. In general, the interactions are more in EG+FA system due to the presence of strong proton-acceptor group (>C=O) and proton-donor group (–NH2), and the interactions are expected to decrease with increase of –CH3 group attached to nitrogen atom. But in the present study, the interaction is strong in EG+DMA system, and it follows the order EG+DMA > EG+DMF > EG+FA. This is due to one of the favorable effects of fitting of smaller molecules into the voids created by the bigger molecules. This geometrical fitting of smaller molecules into the bigger molecules was also reported by the others for interpreting negative \( V_{\text{m}}^{\text{E}} \) vales [28, 30, 31]. Similar type of studies were also reported by other researchers as alcohol one of the component [32, 33].
The variation of deviation in viscosity, Δη, with the mole fraction of EG is shown in Fig. 2. Generally, negative values of Δη indicate the presence of dispersion forces or mutual loss of specific interactions in molecules operating in the systems arising due to weak intermolecular interactions, and positive values of deviation in viscosity indicate strong specific interactions [34, 35]. The sign and magnitude of Δη depend on the combined effect of factors such as molecular size, shape, and intermolecular forces. The molecules of FA, DMF, and DMA are highly polar µ = 3.37, 3.86, and 3.72 D, respectively, at T = 298.15 K [36], and dipole–dipole interactions between the unlike molecules are also expected to play an important role in determining liquid structure. The observed negative Δη values are due to the dipole–dipole interactions between the unlike molecules which lead to strong interactions in the liquid mixtures. This type of behavior (both \( V_{\text{m}}^{\text{E}} \) and Δη negative) is observed by the several researchers [9, 21, 37].
The variation in excess Gibbs free energy of activation of viscous flow, \( \Delta G^{{ * {\text{E}}}} \) with the mole fraction of secondary alcohols, is shown in Fig. 3. The excess Gibbs free energy of activation of viscous flow is negative over the entire composition range studied [38]. The less negative \( \Delta G^{{ * {\text{E}}}} \) values for EG+DMA system indicate that the interactions are predominant compared to EG+FA and EG+DMF systems. Such type of behavior was also observed by Pikkarainen [37]. This supports the conclusions drawn from \( V_{\text{m}}^{\text{E}} \) and Δη.
The existing molecular interactions in the systems are well reflected in the properties of partial molar volumes. The partial molar volumes \( \overline{V}_{{{\text{m}},1}} \) of component 1 (EG) and \( \overline{V}_{{{\text{m}},2}} \) of component 2 (amides) in the mixtures over the entire composition range have been calculated using the following relations:
where \( V_{1}^{ * } \) and \( V_{2}^{ * } \) are the molar volumes of pure components of EG and amides, respectively. The derivates \( \left( {\frac{{\partial V_{\text{m}}^{\text{E}} }}{\partial x}} \right)_{\text{T,P}} \) in Eqs. (7) and (8) are obtained by differentiating Eq. (5) which lead to the following equations for \( \overline{V}_{{{\text{m}},1}} \) and \( \overline{V}_{{{\text{m}},2}} \):
using the above equations \( \overline{V}_{\text{m,1}}^{\text{E}} \),\( \overline{V}_{\text{m,2}}^{\text{E}} \) have been calculated using
The values of \( \overline{V}_{{{\text{m}},1}} \), \( \overline{V}_{{{\text{m}},2}} \), \( \overline{V}_{{_{\text{m,1}} }}^{\text{E}} \), and \( \overline{V}_{{{\text{m}},2}}^{\text{E}} \) are furnished in Table 3. From this table, the values of \( \overline{V}_{{{\text{m}},1}} \) and \( \overline{V}_{{{\text{m}},2}} \) for both the components in the mixtures are less than their respective molar volumes in the pure state i.e., contraction of volume takes place on mixing EG with amides. These data are also supporting the observed negative values of \( V_{\text{m}}^{\text{E}} \) in all the binary systems. Figures 4 and 5 represent the variation of excess partial molar volumes of EG and FA/DMF/DMA in the binary mixtures, respectively. Examination of Figs. 4 and 5 reveals that indicating strong interactions exist between the unlike molecules. These figures support the conclusions drawn from \( V_{\text{m}}^{\text{E}} \) values.
The dynamic viscosities of the binary liquid mixtures have been calculated using various empirical relations like Grunberg and Nissan, Ubbelohde et al., Katti and Chaudari and Heric and Brewer, and the corresponding interaction parameters are also evaluated. The detailed description of the above empirical relations is already reported in our previous papers [13, 15]. Theoretical values of viscosity of the liquid mixtures calculated using the above equations are given in Table 5. Table 6 presents the values of interaction parameters along with the standard deviations, σ. The estimated values of σ are found to be smaller, which indicate that experimental values of viscosities are well correlated with that obtained from different viscosity models.
Conclusions
The densities, ρ, and viscosities, η, of binary mixtures of EG with formamide, DMF, and DMA have been measured over the entire composition range at 308.15 K. From this experimental data, excess molar volume, \( V_{\text{m}}^{\text{E}} \), deviation in viscosity, Δη, and excess Gibbs free energy of activation of viscous flow, \( \Delta G^{{ * {\text{E}}}} \) have been determined. These deviation/excess properties have been fitted to Redlich–Kister-type polynomial. The observed negative values of deviation/excess properties are due to the addition of amide molecules causes breaking of the hydrogen bonds between EG molecules and the subsequent formation of strong hydrogen bonds between the hydrogen atom of –OH group of glycol molecules and oxygen molecule of >C=O group of amide molecules and fitting of smaller molecules into the voids created by the bigger molecules and also due to the dipole–dipole interactions between the unlike molecules causes the strong specific interactions exist between the unlike molecules. The strength of interactions follows the order EG+DMA > EG+DMF > EG+FA. The experimental viscosity values are compared with the viscosity values obtained from different empirical relations, and these are in good agreement with the experimental values.
References
Marcus Y. Introduction to liquid state chemistry. New York: Wiley-Interscience; 1977.
Kumar DS, Sreekanth K, Rao DK. Molecular interactions in the mixtures of 2-chloroaniline with equimolar mixture of methanol and isopropanol/isobutanol. J Mol Liq. 2007;136:90–3.
Ali A, Soghra H. Molecular interaction study in binary mixtures of dimethyl sulphoxide with 1,2-dichloroethane and 1,1,2,2-tetrachloroethane at 303 K. Indian J Phys. 2002;76:23–8.
Rao TS, Veeraiah N, Rambabu C. Excess volume, viscosity and compressibility of binary mixtures consisting of o-chlorophenol, o-cresol and m-cresol with N,N-diethyl acetamide at different temperatures. Indian J Pure Appl Phys. 2002;40:850–6.
Powell GM. Polyethelene glycol. In: Davidson RL, editor. Hand book of water-soluble gums and resins. New York: McGraw-Hill Book Companies; 1980.
Kinart CM, Klimczak M, Cwklinska A, Kinart WJ. Densities and excess molar volumes for binary mixtures of some glycols in 2-methyoxyethanol at T = (293.15, 298.15 and 303.15) K. J Mol Liq. 2007;135:192–5.
Ali A, Nain AK, Kumar N, Ibrahim M. Density and viscosity of magnesium sulphate in formamide + ethylene glycol mixed solvents. Proc Indian Acad Sci (Chem Sci). 2002;114:495–500.
Kinart CM, Klimczak M. Thermodynamic and structural properties of binary mixtures of some glycols with 2-butoxyethaol at T = (293.15, 298.15 and 303.15) K. J Mol Liq. 2009;148:132–9.
Tsierkezos NH, Molinou IR. Thermodynamic properties of water + ethylene glycol at 283.15, 293.15, 303.15 and 313.15 K. J Chem Eng Data. 1998;43:989–93.
Pal A, Singh YP. Viscosity in water + ethylene glycol dimethyl, +diethylene glycol dimethyl, +triethylene glycol dimethyl, and +tetraethylene glycol dimethyl ethers at 298.15 K. J Chem Eng Data. 1996;41:1008–11.
Riddick JA, Bunger WB, Sakano TK. Organic solvents: physical properties and methods of purification. 4th ed. New York: Wiley-Interscience; 1986.
Parker HC, Parker EW. Densities of certain aqueous potassium chloride solutions as determined with a new pycnometer. J Phys Chem. 1925;29:130–7.
Kondaiah M, Kumar DS, Sreekanth K, Rao DK. Densities and viscosities of binary mixtures of propanoic acid with N,N-dimethylaniline and N,N-diethylaniline at T = (303.15, 313.15, and 323.15) K. J Chem Eng Data. 2012;57:352–7.
Kondaiah M, Kumar DS, Sreekanth K, Rao DK. Ultrasonic velocities, densities, and excess molar volumes of binary mixtures of N,N-dimethyl formamide with methyl acrylate, or ethyl acrylate, or butyl acrylate, or 2-ethyl hexyl acrylate at T = 308.15 K. J Chem Thermodyn. 2011;43:1844–50.
Kondaiah M, Sreekanth K, Kumar DS, Rao DK. Volumetric and viscometric properties of propanoic acid in equimolar mixtures of N,N-dimethyl formamide + alkanols at T/K 5 303.15, 313.15, and 323.15. J Sol Chem. 2013;42:494–515.
Sreekanth K, Kumar DS, Kondaiah M, Rao DK. Volumetric and viscometric study of molecular interactions in the mixtures of some secondary alcohols with equimolar mixture of ethanol and N,N-dimethylacetamide at 308.15 K. Phys B. 2011;406:854–8.
Sreekanth K, Kondaiah M, Kumar DS, Rao DK. Influence of temperature on thermodynamic properties of acid–base liquid mixtures: an ultrasonic, volumetric, and viscometric study. J Therm Anal Calorim. 2012;110:1341–52.
Naidu BVK, Rao KC, Subha MCS. Densities, viscosities and excess properties for binary mixtures of some glycols and polyglycols in n-methylacetamide at 308.15 K. J Chem Eng Data. 2003;48:625–7.
Nain AK. Densities and volumetric properties of (acetonitrile + an amide) binary mixtures at temperatures between 293.15 and 318.15 K. J Chem Thermodyn. 2006;38:1362–70.
Nain AK. Molecular interactions in binary mixtures of formamide with 1-butanol, 2-butanol, 1,3-butanediol and 1,4-butanediol at different temperatures: an ultrasonic and viscometric study. Fluid Phase Equilib. 2008;265:46–56.
Nikam PS, Kharat SJ. Density and viscosity studies of binary mixtures of N,N-dimethylformamide with toluene and methyl benzoate at (293.15, 303.15, 308.15, and 313.15) K. J Chem Eng Data. 2005;50:455–9.
Thirumaran S, Ramesh J. Acoustic and excess thermodynamical studies on 1-alkanols with DMA in cyclohexanone at different temperatures. Rasayan J Chem. 2009;2:733–9.
Redlich O, Kister AT. Algebraic representation of thermodynamic properties and the classification of solutions. Ind Eng Chem. 1948;40:345–8.
Reddy KC, Subramanyam SV, Bhimasenachar J. Thermodynamics of binary liquid mixtures containing cyclohexane Part 1. J Phys Soc Jpn. 1964;19:19559–66.
Krestove GA. Thermodynamics of salvation. Chichester: Ellis-Horwood; 1991.
Ohtaki H, Itoh S, Yamaguchi T, Bratos S. Structure of liquid N,N-dimethyl formamide studied by means of X-ray diffraction. Bull Chem Soc Jpn. 1983;56:3406–12.
Gopal R, Aggarwal S, Aggarwal DK. Excess volumes of some polar and non-polar liquids with N,N′-dimethyl formamide. J Chem Thermodyn. 1976;8:1205–8.
Ali A, Nain AK. Ultrasonic and volumetric study of binary mixtures of benzyl alcohol with amides. Bull Chem Soc Jpn. 2002;75:681–7.
Kim NK, Lee HJ, Choi KH, Yu JA, Yoon CJ, Park J, Choi YS. Substituent effect of N,N-dialkylamides on the intermolecular hydrogen bonding with thioacetamide. J Phys Chem B. 2000;104:5572–8.
Assarson P, Eirich FR. Properties of amides in aqueous solution. I. Viscosity and density changes of amide-water systems: an analysis of volume deficiencies of mixtures based on molecular size differences (mixing of hard spheres). J Phys Chem. 1968;72:2710–9.
Pikkarainen L. Densities and viscosities of binary solvent mixtures of N-methyl acetamide with aliphatic alcohols. J Chem Eng Data. 1983;28:381–3.
Sastry SS, Babu SK, Vishwam T, Tiong HS. Excess parameters for binary mixtures of alkyl benozoates with 2-propanol at different temperatures. J Therm Anal Calorim. 2014;116:923–35.
Venkataramana L, Sreenivasulu K, Sivakumar K, Reddy KD. Thermodynamic properties of binary mixtures containing 1-alkanols. J Therm Anal Calorim. 2014;115:1829–34.
Islam MR, Quadri SK. Ultrasonic velocity and viscosity of binary liquid mixtures. Thermochim Acta. 1987;115:335–44.
Tewari K, Patra C, Chakravortty V. Molecular interactions study on binary liquid mixtures of dimethyl sulphoxide with benzene, carbon tetrachloride and toluene from the excess properties of ultrasonic velocity, density and viscosity. Acoust Lett. 1995;19:53–9.
Ali A, Pandey JD, Soni NK, Nain AK, Lal B, Chand D. Densities, ultrasonic speeds, viscosities and refractive indices of binary mixtures of benzene with benzyl alcohol, benzonitrile, benzoyl chloride and chlorobenzene at 303.15 K. Chin J Chem. 2005;23:377–85.
Pikkarainen L. Densities and viscosities of binary mixtures of N,N-dimethyl acetamide with aliphatic alcohols. J Chem Eng Data. 1983;28:344–7.
Ali A, Nain AK, Chand D, Ahmad R. Volumetric, ultrasonic, viscometric and refractive index behavior of binary mixtures of 2,2,4-trimethylpentane with aromatic hydrocarbons: an experimental and theoretical study. J Mol Liq. 2006;128:32–41.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kondaiah, M., Sreekanth, K., Kumar, D.S. et al. Densities, viscosities, and excess properties for binary mixtures of ethylene glycol with amides at 308.15 K. J Therm Anal Calorim 118, 475–483 (2014). https://doi.org/10.1007/s10973-014-4019-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-4019-5