Abstract
Two pyridine carboxaldehyde-derived schiff bases and their copper, nickel and manganese complexes have been synthesized, characterized by spectroscopic and thermal analyses. For the antimicrobial activity experiments, the microorganisms B. megaterium, K. pneumoniae, E. coli, P. aeruginasa, S. aureus, B. subtilis and E. aerogenes have been used. Nickel and copper complex compounds of ligand L2 were recorded as the most effective coordination compounds as antimicrobial. Catalytic activity results revealed that the both manganese and nickel complexes were moderately more effective for the oxidation reactions of cyclohexene and styrene.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Schiff base ligands and their transition metal complexes have captivated the interest of many researches, especially in the application for catalytic reactions including alkane and alkene oxidations [1–4]. Many coordination compounds of different Schiff bases contain different donor sites (e.g., N2O, N4, N2S) in heterocyclic rings, and because of the nature of donor atoms and central metal ions, the transition metal complexes of different Schiff base ligands developed interesting pharmacological activities such as antibacterial, antiviral, antimalarial, antituberculosis and antitumoural agents [5–7]. So far, various pyridine carboxaldehyde-derived Schiff bases and complexes have been synthesized, characterized and examined for their different biological activity [8–10], and recently, some transition metal complexes of these kinds of Schiff bases have been found to be an acetylcholinesterase inhibitor [11]. In addition to above researches, various pyridine-derived Schiff bases and their different complexes have also been examined for their catalytic activities in different organic reactions [12–14]. Our work deals with the synthesis, characterization and thermal, antimicrobial and catalytic features of pyridine-2-carboxaldehyde-derived Schiff bases and their metal complexes.
Experimental
Pyridine-2-carboxaldehyde, o-diaminobenzene, 1,2-diaminocyclohexane and acetate salts of copper(II), nickel(II) and manganese(II) were purchased from Sigma-Aldrich. Nuclear magnetic resonance spectra were recorded on a Bruker AV 300 MHz spectrometer in the solvent CDCl3. Infrared spectra were obtained using KBr disks on a Shimadzu 8300 FTIR spectrophotometer in the region of 400–4000 cm−1. Ultraviolet spectra were run in ethanol on a Shimadzu UV-160 A spectrophotometer. Mass spectra of the ligand were recorded on a LC/MS APCI AGILENT 1100 MSD spectrophotometer. The oxidation products were analyzed with a gas chromatograph (Shimadzu, GC-14B) equipped with a SAB-5 capillary column and a flame ionization detector. Elemental analyses were performed on a LECO CHNS 932 elemental analyzer, and the metal analyses were carried out on an Ati Unicam 929 Model AA Spectrometer in solutions prepared by decomposing the compounds in aqua regia and subsequently digesting them in conc. HCl. Thermal analyses of synthesized ligand and its metal complexes were carried out on a Perkin-Elmer Thermogravimetric Analyzer TG/DTA 6300 instrument under nitrogen atmosphere between the temperature range 30 and 850 °C at a heating rate of 10 °C min−1. Magnetic measurements were taken by the Gouy method using Hg[Co(SCN)4] as calibrant. Molar conductances of the Schiff base ligands and their transition metal complexes were determined in MeOH (ca. 10−3 M) at room temperature using a Jenway Model 4070 conductivity meter.
Synthesis of the ligands
The two ligands were synthesized from 1:2 equilibrium of the diamine and the pyridine carboxaldehyde. For ligand L1, 2 mmol (0.214 g) pyridine-2-carboxaldehyde and 1 mmol (0.114 g) o-diaminohexane were mixed in 30 mL ethanol and refluxed for 8 h, and for the ligand L2, 2 mmol (0.214 g) pyridine-2-carboxaldehyde and 1 mmol (0.108 g) o-diaminobenzene were mixed in 30 mL ethanol and refluxed for overnight. The resulted precipitates were recrystallized from ethanol. Synthesis scheme can be seen in Fig. 1.
L1 (N 1 E,N 2 E)-N 1 ,N 2-bis(pyridin-2-ylmethylene)cyclohexane-1,2-diamine
Chemical formula: C18H20N4. Yield: 75 %, m.p.: 131 °C, Elemental Analysis found % (calculated %): C 74.3(73.9) H 6.1(6.9) N 19.3(19.2). UV–Vis (ethanol) (λmax, nm): 235, 266 FT-IR (KBr, cm−1): 1644 (C=N); 1586, 1466 (Py, Ar–H); 2859, 2926 (CH2); 617, 666 (Py). Mass spectrum (LC/MS APCI): m/z 293.2 [M + H]+. 1H NMR(300 MHz, CDCl3): δ 8.53 (2H-1′, dd, J = 3 Hz, 1 Hz), 7.2 (2H-2′, ddd, J = 6.1 Hz, 3 Hz, 1 Hz), 7.63 (2H-3′, dt, J = 8 Hz, 1.7 Hz), 7.87 (2H-4′, dd, J = 8 Hz, 1 Hz), 3.53 (2H-1, m), 1.5 (2Ha-2, m), 1.81 (4H-3, 2Hb-2, m), 8.3 (CH=N, s). 13C NMR (300 MHz, CDCl3): δ 73.5 (C-1), 32.7 (C-2), 24.3 (C-3), 149.2 (C-1′), 124.5 (C-2′), 136.4 (C-3′), 121.3 (C-4′), 154.6 (C-5′), 161.4 (CH=N).
L2 (N 1 E,N 2 E)-N 1,N 2-bis(pyridin-2-ylmethylene)benzene-1,2-diamine
Chemical formula: C18H14N4. Yield: 63 %, m.p.: 121 °C, Elemental Analysis found % (calculated %): C 74.9(75.5) H 5.1(4.9) N 19.6(19.6). UV–Vis (ethanol) (λmax, nm): 234, 275, 309. FT-IR (KBr, cm−1): 1622 (C=N); 1591, 1500, 1448, 3055 (Py, Ar–H); 2673, 2851, 2920 (CH2), 621 (Py). Mass spectrum (LC/MS APCI): m/z 287.1 [M + H]+. 1H NMR (300 MHz, CDCl3): δ 8.48 (2H-1′, d, J = 8 Hz), 7.25 (2H-2′, t, J = 8 Hz), 7.8 (2H-3′, dt, J = 7.8 Hz, 1.7 Hz), 7.87 (2H-4′, dd, J = 7.1, 1 Hz), 6.3 (2H-2, s), 6.89 (2H-3, d, J = 8 Hz), 8.48 (CH=N, overlapped with H-1′). 13C NMR (300 MHz, CDCl3): δ 142.7 (C-1), 120.1 (C-2), 121.0 (C-3), 149.2 (C-1′), 124.6 (C-2′), 136.8 (C-3′), 122.3 (C-4′), 150.3 (C-5′), 157.4 (CH=N).
Synthesis of the complex compounds
Acetate salts (1 mmol) of Cu(II), Co(II) and Ni(II) were mixed with ethanol solution of our ligand (1 mmol). The resulting mixtures were left refluxing overnight. Complex compounds were crystallized from ethanol.
Cu(L1)(OAc)2
(C22H26CuN4O4), yield: 60 %, m.p.: 167.4 °C, Elemental Analysis found % (calculated %): C 55.4 (55.7), H 5.4 (5.5), N 11.9 (11.8), Cu 13.7 (13.4). UV–Vis (ethanol) (λmax, nm): 264, 348, 501. FT-IR (KBr, cm−1): 1628 (C=N), 1472, 3075 (Py, Ar–H), 2932(CH2), 438 (M–N), 1595 (C–O), 759 (COO), 693 (Py). ΛM (Ω−1 cm2 mol−1): 7.1. µeff (B.M.): 1.98.
Mn(L1)(OAc)2
(C22H26MnN4O4), yield: 59 %, m.p.: 147.1 °C, Elemental Analysis found % (calculated %): C 56.9 (56.8), H 5.8 (5.6), N 11.9 (12.0), Mn 12.4 (11.8). UV–Vis (ethanol) (λmax, nm): 265, 339, 510. FT-IR (KBr, cm−1): 1629 (C=N), 1416, 3020 (Py, Ar–H), 2934, 2862 (CH2), 413 (M–N), 1577 (C–O), 781 (COO), 632, 686 (Py). ΛM (Ω−1 cm2 mol−1): 12. µeff (B.M.): 5.85.
Ni(L1)(OAc)2
(C22H26NiN4O4), yield: 65 %, m.p.: 155.2 °C, Elemental Analysis found % (calculated %): C 56.7 (56.3), H 6.0 (5.6), N 12.1 (11.9), Ni 12.6 (12.5). UV–Vis (ethanol) (λmax, nm): 246, 341, 468. FT-IR (KBr, cm−1): 1618 (C=N), 1408, 3015 (Py, Ar–H), 2924, 2859 (CH2), 440 (M–N), 1557 (C–O), 759 (COO), 630, 684 (Py). ΛM (Ω−1 cm2 mol−1): 9.3. µeff (B.M.): 3.15.
Cu(L2)(OAc)2
(C22H20CuN4O4), yield: 55 %, m.p.: 148 °C, Elemental Analysis found % (calculated %): C 56.7 (56.5), H 4.5 (4.3), N 12.1 (11.9), Cu 13.8 (13.6). UV–Vis (ethanol) (λmax, nm): 238, 268, 365, 482. FT-IR (KBr, cm−1): 1600 (C=N), 1428, 1481, 3059 (Py, Ar–H), 2927 (CH2), 441 (M–N), 1568 (C–O), 748 (COO), 616, 675 (Py). ΛM (Ω−1 cm2 mol−1): 11.7. µeff (B.M.): 1.95.
Mn(L2)(OAc)2
(C22H20MnN4O4), yield: 55 %, m.p.: 173.6 °C, Elemental Analysis found % (calculated %): C 57.9 (57.5), H 4.6 (4.4), N 12.5 (12.2), Mn 12.3(11.9). UV–Vis (ethanol) (λmax, nm): 240, 271, 309, 517. FT-IR (KBr, cm−1): 1598 (C=N), 1476, 1411, 3054 (Py, Ar–H), 2919 (CH2), 501 (M–N), 1563 (C–O), 743 (COO), 614, 650 (Py). ΛM (Ω−1 cm2 mol−1): 13.5. µeff (B.M.): 5.90.
Ni(L2)(OAc)2
(C22H20NiN4O4), yield: 54 %, m.p.: 131.2 °C, Elemental Analysis found % (calculated %): C 57.2 (57.0), H 4.7 (4.4), N 12.3 (12.1), Ni 12.9 (12.7). UV–Vis (ethanol) (λmax, nm): 242, 340, 379, 448. FT-IR (KBr, cm−1): 1595 (C=N), 1481, 1410, 3063 (Py, Ar–H), 2972 (CH2), 445 (M–N), 1539 (C–O), 744 (COO), 661 (Py). ΛM (Ω−1 cm2 mol−1): 7.6. µeff (B.M.): 3.11.
Oxidation procedure
A mixture of 1 × 10−3 mol catalyst, 20 mL solvent (CH3CN) and 10 mmol cyclohexene/styrene was stirred under nitrogen atmosphere in a 50-mL round-bottom flask. Then, 20 mmol hydrogen peroxide (30 % in water) was added dropwise. The resulting mixture was vigorously stirred for 8 h at 60 °C. After filtration and washing with solvent, the filtrate was concentrated and then subjected to GC analysis. The yields were recorded as the GC yield based on the starting styrene or cyclohexene. The identity of the oxidation products was confirmed by GC–MS.
Preparation of microorganism culture
The test bacteria kept at 4 °C were injected into Nutrient Buyyon and incubated at 37 °C for 24 h, and the yeasts were incubated in Sabouraud dextrose agar for 24 h at 30–103 °C. Mueller–Hinton agar (MHA) (Oxoid) and Sabouraud dextrose agar (SDA) sterilized in a flask and cooled to 45–50 °C were distributed to sterilized petri dishes with a diameter of 9 cm (15 mL) after injecting cultures (0.1 mL) of bacteria and yeast (106 bacteria per mL and 105 yeasts per mL), and distributing medium in petri dishes homogenously. Dishes injected with extracts were located on the solid agar medium by pressing slightly. Petri dishes were kept at 4 °C for 2 h; placks injected with yeasts were incubated at 25 °C; and the bacteria were incubated at 37 °C for 24 h. At the end of the period, inhibition zones formed on the MHA and SDA were evaluated in millimeters.
Results and discussion
Spectral analysis
Examination of 1H NMR spectrum of L1 revealed imine protons (CH=N) as broad singlet at δ 8.3. The aromatic protons next to nitrogen in the pyridine ring (2H-1′) were seen further downfield at δ 8.53 as dd (J = 1, 3.0 Hz). 2H-4′ was at 7.87 as dd (J = 8, 1 Hz), next to this signal at δ 7.63 was a dt (2H-3′, J = 8, 1.7 Hz). The two hydrogens of 2′ para to the imine group were seen furthest upfield at 7.20 ppm as ddd (J = 3, 6, 1 Hz), coupling with H-1′, H-3′ and H-4′. As for the cyclohexane ring, the most deshielded protons were 2H-1 (3.53 ppm, m). Six hydrogens belonging four hydrogens of 3 and two hydrogens of 2 on the cyclohexane ring were seen at 1.81 ppm, and we suspect that the other two hydrogens of 2 (2Ha-2) fall into slight shielding of the lone pair of nitrogen in the imine group or/and the pyridine rings and show a multiplet at 1.50 ppm [15]. 1H NMR of L2 [14] revealed the aromatic protons, 2H-2, as a singlet at 6.3 ppm. The other two hydrogens (2H-3) on the benzene ring gave a broad doublet (J = 8 Hz) at 6.89 ppm. As for the pyridine ring, 2H-1′ gave a doublet (J = 8 Hz) at 8.48 ppm. H-2′ was observed at 7.25 ppm as a triplet (J = 8 Hz). The imine protons were overlapped with the H-1′ protons on the pyridine ring at 8.48 ppm. 13C NMR spectra of the ligands gave the imine carbon frequencies at 157.4 ppm for L2 and at 161.4 ppm for L1. Cyclohexane carbons in ligand L1 were at δ 24.3 (C-3), δ 32.7 (C-2) and at 73.5 (C-1), which is the most deshielded because of the nitrogen in the imine group. Pyridine ring carbons for this ligand were seen between 121.3 and 161.4 ppm. All the aromatic carbons belonging L2 were observed between 120.1 and 157.4 ppm. The spectral data can be seen in the experimental part.
IR spectra of the ligands revealed the imine stretching frequencies at 1644 cm−1 (L1) and at 1622 cm−1 (L2). These imine frequencies were considerably redshifted in all of the complexes, which proves the chelation with the metal atoms. The ligands showed pyridine stretching before chelation at 1586 cm−1, 1466 cm−1 (L1) and at 1591 cm−1, 1448 cm−1 (L2); however, all the complexes gave these stretching at bigger wave lengths, respectively, lower wave numbers, frequencies. Additionally, the other known pyridine in plane bendings for the ligands was observed between 616 and 675 nm, which were shifted toward lower or higher frequencies in the spectra of all the complexes [15, 16]. For these kinds of complex compounds, the values of asymmetric and symmetric vibrations of COO− are important. The IR spectra of all the complexes revealed that asymmetric vibrations [νas(COO−)] were observed between 1539 and 1578 cm−1, whereas symmetric vibrations [νs(COO−)] were observed between 1332 and 1342 cm−1 range [17, 18]. The sharp bands between 743 and 781 cm−1 are known to be the characteristic bands for monodentate acetates [17].
UV–visible spectra of the ligands and the complexes showed absorption bands between 234 and 517 nm. The spectra of the complexes showed some bands in the high-energy region at 309–379 nm which can be assigned to charge-transfer L-M bands [19, 20]. The complex compounds showed dd transitions between 441 and 517 nm.
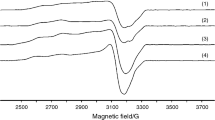
Magnetic susceptibility studies revealed the geometries of all the complexes to be octahedral. For the copper complexes, the magnetic moments were calculated as 1.95 and 1.98 B.M. [17]. The manganese complexes gave these values as 5.85 and 5.95 B.M. [21]. The nickel complexes also revealed their characteristic magnetic moment values as 3.11 and 3.15 B.M., suggesting octahedral geometry [21, 22]. Molar conductivities of the ligand and its coordination compounds were between 7.1 and 13.5 Ω−1 cm2 mol−1, which proves their nonelectrolyte features [23].
Thermal analysis
Examination of TG graphics of L1 gives two-step degradation process starting at 70 °C, and the biggest loss was seen at 306 °C with 52.73 % (Calc. 53.4 %), corresponding the two pyridine units. One can divide the TG graphics of the complexes of L1 into three degradation steps, the first one of which results in loosing the two acetate units. The second and the third steps of degradations in the TG graphics of the coordination compounds Ni(L1)(OAc)2 and Mn(L1)(OAc)2 are assigned to C8H12N2 (benzene and imine moieties) and 2(C5H4N) (two pyridine moieties), respectively. The copper complex of the same ligand gave the second degradation with a total mass loss of 15.76 % (Calc. 16.03 %), corresponding the benzene moiety only, and the second and the biggest lost occured over 440 °C with 43.8 % (Calc. 44.3 %), corresponding the pyridine and the imine moieties (2 × C6H5N2).
TG graphics of E6 also gave two steps of degradations: The first step was the loss of benzene [27.42 % (Calc. 26.54 %)], and the second step was recorded and calculated as the pyridine and the imine groups [72.50 % (Calc. 73.34 %)]. All the complexes of L1 gave three degradation steps: The first of which corresponds to the loss of two acetates, and the second and the third steps belong to the losses of benzene ring and the pyridines with imine groups, respectively.
Among the whole of the complex compounds, the nickel(II) complex of L1 and the manganese(II) complex of L2 were more temperature-stable compared to the others, and all the metal complexes gave their metal percentages as their residual parts. The thermal data can be seen in Table 1, and the Figs. 2 and 3 show the thermal graphics of the synthesized compounds.
Catalytic activity
Both styrene and cyclohexene oxidation reactions gave moderately good results. Among the complex compounds, nickel(II) complex of L1 and manganese(II) complex of L2 showed highest performances for these oxidation reactions as catalysts. Since the oxidation reactions are carried out in high temperatures, the performances of these complexes might also be related to the fact that they were thermally the most stable catalysts. The highest performance as a catalyst belongs to the Mn(L2)(OAc)2 with a styrene conversion percentage of 71.2. For the same reaction, nickel complex of L1 also showed good conversion with a percentage of 64.8 and resulted in 35 % styrene oxide selectivity. For the cyclohexene oxidation reactions, moderate conversions were observed for all the coordination compounds. Nonetheless, highest conversions among them belong to nickel complex of L1 and manganese complex of L2 with percentages of 41.7 and 55.6, respectively, and the cyclohexene oxide selectivities for these complexes were recorded as 20.9 and 30.1 %. For cyclohexene oxidation reaction, moderately highest selectivities were recorded for 2-cyclohexene-1-ol. The rest of the data can be seen in Tables 2 and 3, and the oxidation reaction schemes are given in Fig. 4.
Antimicrobial activity
All the synthesized compounds were examined for their antimicrobial activity against a number of microorganisms. Both ligands L1 and L2 were slightly effective against the microorganisms, however, all the complex compounds gave much more effective results compared to their ligands. Between the synthesized ligands, ligand L2 was slightly more effective toward all microorganisms than L1. It is obvious from the results that the coordination compounds of L2 gave better results than those of L1 (Tables 4, 5). Especially, nickel and copper complexes of ligand L2 gave bigger inhibition zones than those of other complexes. The biggest inhibition zones with around 18 mm belong to the copper complexes of both ligands against P.aeruginosa, E. aerogenes and B. megaterium. Among the complex compounds, nickel complex of L1 was the least effective against most of the organisms. All the inhibition zones for this complex were recorded between 12 and 14 mm, and the latter is the biggest zone seen for the microorganisms P.aeruginosa and E.aerogenes. Antimicrobial data are given in Tables 4 and 5.
Conclusions
The present work describes the synthesis and characterization of two pyridine-2-carboxaldehyde-derived Schiff bases and their copper(II), nickel(II) and manganese(II) complexes. All spectral data and the thermal analysis supported the proposed structures of all synthesized compounds. Magnetic moment values strongly suggested the geometries of the coordination compounds to be octahedral. Catalytic activity results showed nickel and manganese complexes of the ligands to be better catalysts toward oxidation reactions of styrene and cyclohexene. All the coordination compounds were better antimicrobial agents compared to their ligands.
References
Ceyhan G, Köse M, McKee V, Urus S, Gölcü A, Tümer M. Tetradentate Schiff base ligands and their complexes: synthesis, structural characterization, thermal, electrochemical and alkane oxidation. Spectrochim. Acta A. 2012;95:382–98. doi:10.1016/j.saa.2012.04.001.
Heshmatpour F, Rayati S, Hajiabbas MA, Abdolalian P, Neumüller B. Copper(II) Schiff base complexes derived from 2,20-dimethyl-propandiamine: synthesis, characterization and catalytic performance in the oxidation of styrene and cyclooctene. Polyhedron. 2012;31:443–50. doi:10.1016/j.poly.2011.09.048.
Islam SkM, Roy A, Dalapati S, Saha R, Mondal P, Ghosh K, Chatterjee S, Sarkar K, Guchhait N, Mitra P. Synthesis, crystal structure and spectroscopic studies of a cobalt(III) Schiff base complex and its use as a heterogeneous catalyst for the oxidation reaction under mild condition. J Mol Catal A Chem. 2013;380:94–103. doi:10.1016/j.molcata.2013.09.022.
Ceyhan G, Celik C, Urus S, Demirtas I, Elmastas M, Tümer M. Antioxidant, electrochemical, thermal, antimicrobial and alkane oxidation properties of tridentate Schiff base ligands and their metal complexes. Spectrochim Acta A. 2011;81:184–98. doi:10.1016/j.saa.2011.05.106.
West DX, Liberta AE, Padhye SB, Chikate RC, Sonawane PB, Kumbhar AS, Yerande RG. Thiosemicarbazone complexes of copper(II): structural and biological studies. Coord Chem Rev. 1993;123:49–71.
Badea M, Calu I, Chifiriuc MC, Bleotu C, Marin A, Ion S, Ioniţă G, Stanică N, Măruţescu L, Lazăr V, Marinescu D, Olar R. Thermal behaviour of some novel antimicrobials based on complexes with a Schiff base bearing 1,2,4-triazole pharmacophore. J Therm Anal Calorim. 2014;118:1145–57. doi:10.1007/s10973-014-3821-4.
Zayed EM, Zayed MA, Hindy AMM. Thermal and spectroscopic investigation of novel Schiff base, its metal complexes, and their biological activities. J Therm Anal Calorim. 2014;116:391–400. doi:10.1007/s10973-013-3560-y.
Omyma AMA, Samir M, El-Medani SM, Doaa AA, Doaa AN. Metal carbonyl complexes with Schiff bases derived from 2-pyridinecarboxaldehyde: Syntheses, spectral, catalytic activity and antimicrobial activity studies. J Mol Struct. 2014;1074:713–22. doi:10.1016/j.molstruc.2014.05.035.
Ramírez-Jiménez A, Luna-García R, Cortés-Lozada A, Hernández S, Ramírez-Apan T, Nieto-Camacho A, Gómez E. Dinuclear heptacoordinate dibutyltin(IV) complexes derived from Schiff bases and dicarboxylates: synthesis, cytotoxicity, and antioxidant activity. J Organomet Chem. 2013;738:10–9. doi:10.1016/j.jorganchem.2013.03.038.
Patel RN, Singh A, Sondhıya VP, Singh Y, Shukla KK, Patel DK, Pandey R. Synthesis, characterization, and biological activity of nickel(II) complexes with a tridentate Schiff base derived from heterocyclic aldehyde. J Coord Chem. 2012;65(5):795–812. doi:10.1080/00958972.2012.662592.
Kopel P, Dolezal K, Langer V, Jun D, Adam V, Kuca K, Kizek R. Trithiocyanurate complexes of Iron, Manganese and Nickel and their Anticholinesterase activity. Molecules. 2014;19:4338–54. doi:10.3390/molecules19044338.
Sanmartín-Matalobos J, Portela-García C, García-Deibe AM, Fondo M, Briones-Miguéns L. Tuning the ring-chain tautomerism of a tetrahydroquinazoline/Schiff base system with unexpected methanol oxidation. Polyhedron. 2013;65:181–6. doi:10.1016/j.poly.2013.08.034.
Gomes AC, Bruno SM, Gago S, Lopes RP, Machado DA, Carminatti AP, Valente AA, Pillinger M, Gonçalves IS. Epoxidation of cyclooctene using soluble or MCM-41-supported molybdenum tetracarbonylepyridylimine complexes as catalyst precursors. J Organomet Chem. 2011;696:3543–50. doi:10.1016/j.jorganchem.2011.07.040.
Makhubela BCE, Jardine A, Smith GS. Pd nanosized particles supported on chitosan and 6-deoxy-6-amino chitosan as recyclable catalysts for Suzuki-Miyaura and Heck cross-coupling reactions. Appl Catal A Gen. 2011;393:231–41. doi:10.1016/j.apcata.2010.12.002.
Lu X-H, Xia Q-H, Zhan H-J, Yuan H-X, Ye C-P, Su K-X, Xu G. Synthesis, characterization and catalytic property of tetradentate Schiff-base complexes for the epoxidation of styrene. J Mol Catal A Chem. 2006;250:62–9. doi:10.1016/j.molcata.2006.01.055.
Shaki M, Azam M, Azim Y, Parveen S, Khan AU. Synthesis and physico-chemical studies on complexes of 1,2-diaminophenyl-N, N0-bis-(2-pyridinecarboxaldimine), (L): a spectroscopic approach on binding studies of DNA with the copper complex. Polyhedron. 2007;26:5513–8. doi:10.1016/j.poly.2007.08.032.
El-Seıdy AMA. In situ room temperature synthesis and characterization of Salicylaldehyde phenylhydrazone metal complexes, their cytotoxic activity on MCF-7 Cell Line, and Their Investigation as Antibacterial and Antifungal Agents. Synth. React Inorg Me. 2015;45:437–46. doi:10.1080/15533174.2013.841209.
Dojer B, Pevec A, Šegedin P, Jaglicˇic´Z, Stropnik C, Kristl M, Drofenik M. Cobalt(II) coordination compounds with acetate and 2-aminopyridine ligands: synthesis, characterization, structures and magnetic properties of two polymorphic forms. Inorg Chim Acta. 2010;363:1343–7. doi:10.1016/j.ica.2009.12.052.
Ispir E. The synthesis, characterization, electrochemical character, catalytic and antimicrobial activity of novel, azo-containing schiff bases and their metal complexes. Dyes Pigments. 2009;82:13–9. doi:10.1016/j.dyepig.2008.09.019.
Kurtoglu M, Ispir E, Kurtoglu N, Toroglu S, Serin S. New soluble coordination chain polymers of nickel(II) and copper(II) ions and their biological activity. Transit Metal Chem. 2005;30:765–70. doi:10.1007/s11243-005-6273-7.
Shennar KA, Butcher RJ, Greenaway FT. Co(II), Cu(II), Mn(II) and Ni(II) complexes of maleic hydrazide. Inorg Chim Acta. 2015;425:247–54. doi:10.1016/j.ica.2014.09.030.
El-Sayed AEM, Al-Fulaij OA, Elaasar AA, El-Defrawy MM, El-Asmy AA. Spectroscopic characterization and biological activity of dihydrazone transition metal complexes: crystal structure of 2,3-butanedione bis(isonicotinylhydrazine). Spectrochim Acta A. 2015;135:211–8. doi:10.1016/j.saa.2014.07.006.
Tümer M, Çelik C, Köksal H, Serin S. Transition metal complexes of bidentate Schiff base ligands. Transit Metal Chem. 1999;24:525–32.
Acknowledgements
We would like to thank K. Maras Sutcu Imam University Research Projects Coordination Unit for the financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bal, S., Orhan, B., Connolly, J.D. et al. Synthesis and characterization of some Schiff bases, their metal complexes and thermal, antimicrobial and catalytic features. J Therm Anal Calorim 121, 909–917 (2015). https://doi.org/10.1007/s10973-015-4617-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-4617-x