Abstract
This study focused on preparation and thermal properties of poly(stearyl methacrylate) shell (PSMA) microcapsules containing n-octadecane as a phase change material (PCM). Pentaerythritol triacrylate (PETA) and divinylbenzene (DVB) were employed as crosslinking agents. The surface morphologies, particle sizes, and distributions of the microencapsulated phase change material (microPCM) were studied by scanning electron microscopy. The thermal properties, thermal reliabilities, and thermal stabilities of the microPCMs were investigated by differential scanning calorimetry and thermal gravimetric analysis. The microPCM with DVB exhibits higher phase change enthalpies of melting (87.9 J g−1) and crystallization (94.8 J g−1) and a greater thermal stability in comparison with the microPCM with PETA. The phase change temperatures and enthalpies of the microPCMs varied little after thermal cycles. Thermal images showed that the gypsum board with PSMA/n-octadecane microPCM possessed temperature-regulated property. Therefore, microencapsulated n-octadecane with PSMA as shell has good thermal energy storage and thermal regulation potential.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phase change material (PCM) has received wide spread attention for thermal energy storage or thermal regulation because they can store or release thermal energy at nearly constant temperature and give a large thermal storage capacity via phase change [1, 2]. As a typical organic PCM with desirable advantages, such as high heat of fusion, safe, chemically inert, non-corrosive, and little or no supercooling [2, 3], n-alkane is attractive for the application in energy-saving building material. Microencapsulation is one of the most effective techniques for the incorporation of n-alkane in building structures. With microencapsulation, the PCM is packed in a tiny container made by polymer or inorganic as shells [4], which has the size of 1–1,000 µm. Thus, the hard shell can prevent the interaction between the core materials and the matrix materials. Furthermore, the small size of microencapsulation PCM (microPCM) can provide a better surface/volume ratio, which can increase the heat transfer rate. In addition, microencapsulation of PCM can bring some more advantage of avoiding leakages and tolerating volume change as the phase change occur [5, 6], and reducing the reactivity of the core materials toward the external environment [7].
The performances of the shell materials play a significant role in altering the properties of the microPCMs including surface morphologies, thermal storage capacities, and thermal stabilities. Several natural and synthetic polymers have been used as shell materials to microencapsulate PCM, such as silk fibroin/chitosan [8, 9], gelatin/acacia [10, 11], melamine-formaldehyde [8, 12], urea–formaldehyde [13, 14], polystyrene [15, 16], polyurethane [17, 18], and acrylic resin polymers [4, 19–29]. Being an environmentally friendly polymer with certain desirable properties of nontoxic, chemical resistance, and a relatively good mechanical strength, the acrylic resin has received a growing attention in microencapsulation of n-alkane [4, 19–29]. Alkan et al. selected polymethylmethacrylate (MMA) as shell to microencapsulate docosane [4], n-eicosane [19], n-octacosaneand [20], and n-hexadecane [21] via an emulsion polymerization. Chang et al. [22] introduced a PMMA network-silica hybrid as the shell material to synthesize n-octadecane-containing microcapsule based on a suspension polymerization. Ma et al. [23] proposed a UV irradiation-initiated methylmethacrylate (MMA) polymerization to fabricate microcapsules containing paraffin. Shan et al. [24] reported a microencapsulation of n-octadecane with poly(methacrylic acid (MAA)-co-MMA) as the microencapsulated material. Sánchez et al. [25] used methyl acrylate (MA), MAA to copolymerize with MMA for preparing PRS paraffin-containing microcapsules. Ma et al. [26] described a microencapsulation of binary core materials in the poly(MMA-co-divinylbenzene(DVB)) spheres. In our previous work [27], n-octadecane was microencapsulated by a series of MMA-based polymer shells. However, the research work of acrylic resin microcapsules containing PCM has essentially focused on using MMA as the dominant constituent in the shell material composition; relatively few papers are devoted to the studies of other acrylic to microencapsulate the PCM. Yang et al. [28] utilized polystyrene, PMMA, and polyethyl methacrylate (PEMA) as shell materials to prepare tetradecane-containing microcapsules by an in situ polymerization. Their result indicated that the soft polymer material might be favorable to microencapsulate the n-alkane. Alay et al. [29] fabricated n-hexadecane-containing microcapsules with poly(n-butyl acrylate) (PBA) as the shell materials through an emulsion polymerization.
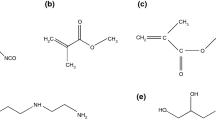
Stearyl methacrylate (SMA) is a kind of long-chain methacrylate ester obtained from plant oils, which are an important renewable raw materials for chemistry industry and attractive from an environmental perspective [30, 31]. Due to the long alkyl side chains of SMA, SMA has a good affinity to n-alkane, and PSMA is softer than PEMA. In addition, the long-chain alkyl acrylate polymers have a tendency to be more ordered crystalline structures [32]. In this case, the thermal resistant temperatures of microPCMs with these polymers as shell materials are highly likely to increase. However, based on the literature survey above, microencapsulation of PCM by PSMA as shell is rarely reported. Therefore, the objective of this study was to microencapsulate PCM by PSMA as shell and n-octadecane as core for thermal energy storage and thermal regulation. Furthermore, pentaerythritol triacrylate (PETA) and DVB were used as crosslinking agents to provide a more stable morphology and a greater thermal stability of the microPCM. The chemical structures of SMA, PETA, and DVB are shown in Fig. 1. Subsequently, the properties of the crosslinked PSMA/n-octadecane microPCMs including the surface morphologies, heat capacities, thermal stabilities, thermal reliabilities, and temperature-regulated properties were investigated in detail.
Experimental
Materials
Stearyl methacrylate (SMA, Aladdin Chemistry Co., Ltd, purity, 99 mass%) and n-octadecane (Alfa, purity, 99 mass%) were used as the shell forming monomer and the core material, respectively. Divinylbenzene (DVB, Aladdin Chemistry Co.,Ltd, purity about 80 mass%) and pentaerythritol triacrylate (PETA, Nanjing Shoulashou Co.,Ltd, purity about 80 mass%) were employed as the crosslinking agents. 2,2′-Azobisisobutyronitrile (AIBN, Shanghai Jingchun Chemical Co., Ltd, purity 98 mass%) was used as an initiator. Sodium salt of styrene-maleic anhydride polymer (Shanghai Leather Chemical Works) was selected as a dispersant.
Preparation of microcapsules
Preparation of microPCMs
MicroPCMs containing n-octadecane were fabricated by a suspension-like polymerization method. This process usually involves the mixture of monomers and initiators, mainly as a discontinuous phase, into a continuous phase generally containing of water consisting small amount of suspension stabilizer, which was described in detail in [27]. In the case of n-octadecane microencapsulation, the discontinuous phase contained n-octadecane (10.0 g), SMA (7.0 g), crosslinking agent (3 g), and AIBN (0.3 g). While the preparation of copolymer without n-alkane was conducted using the similar method excepting the discontinuous without n-octadecane. Once obtained, the microcapsules or polymer particles were separated by centrifugation and filtration and then repeatedly washed with 30 mass% ethanol solution at about 50 °C. Finally, the samples were dried in an oven at 45 °C for 24 h.
Characterization of microcapsules
The surface features of the polymer particles and the microPCM particles were observed using a field emission scanning electron microscope (FESEM, HITACHI S4800). All samples were sprayed with a layer of gold before the observation. More than 200 microPCM particles were counted on the SEM micrographs to measure the diameters of the as-prepared microcapsules.
Measurement of melting/crystallization temperatures and melting/crystallization enthalpies of the microPCMs and n-octadecane was performed in a differential scanning calorimetry (DSC, METTLER TOLEDO DSC 823E). These measurements were conducted varying the temperature in the range from 0 to 50 °C at a scanning rate of ±5 °C min−1. An aluminum crucible of 40 µL under an argon atmosphere flow of 60 mL min−1 was used.
In order to determine thermal reliability of the as-prepared microcapsules, thermal cycling tests (1,000 melting/crystallizing processes) were carrying out using a closed thermal cycling test chamber (BPH-060A, Shanghai Lanbao Machinery and Equipment Co., Ltd, China) according to the experimental procedure described in the literature by Ma et al. [26]. The changes in the thermal properties after 1,000 thermal cycling of the microcapsules were investigated using the above DSC analysis.
The mass loss of the microPCMs and n-octadecane with temperature increasing was evaluated with a thermal gravimetric analysis (TG, METTLER TOLEDOTG/DSC1). The heating rate of 10 °C min−1 was from 50 to 500 °C under an argon atmosphere.
A heating-up experiment was conducted according to the literature [23, 33] on two gypsum boards to investigate the temperature-regulated properties of the as-prepared microcapsules. Board-a contained 15 mass% of PSMA/n-octadecane microPCM with DVB, while board-b is a raw gypsum board employed as a reference. The mass of each gypsum board was about 20.0 g, and the dimension of each of them was 35 mm × 35 mm × 15 mm. Both the gypsum broads with and without PSMA/n-octadecane microPCM were cooled down to 1 °C and then placed in a thermostatic bath keeping at a constant temperature of 70 °C. The temperature distributions of the surface of the gypsum boards were measured using an infrared thermography (FLIR SC600-Series). The screen of the infrared thermography was observed from a distance of 30 cm to the gypsum board at an ambient temperature of 25 °C. Images of the surface of each gypsum board and the change of the surface temperature at the center of the two gypsum boards with the temperature increasing were taken at different times from 2 to 40 °C, which were downloaded by FLIR ResearcherIR software.
Results and discussion
Morphologies of microcapsules
The morphologies of the as-prepared dried polymer particles and microcapsules particles were observed with a scanning electron microscope, as shown in Fig. 2. All the PSMA polymer particles and the PSMA/n-octadecane microPCMs particles have spherical profiles. However, the surface morphologies of these particles are apparently distinguishing. As can be seen from Fig. 2a, b, the morphologies of the PSMA polymer particles with PETA as crosslinking agent resembled those of the P(SMA-co-MMA) copolymer particles with pentaerythritol tetraacrylate (PETRA) in our previous study [34], showing many dimples on the surface of some of them. In contrary, the PSMA polymer particles with DVB have smooth and compact surfaces, showing no dimple on their surfaces. This may suggest that the PSMA polymer particles with DVB have a greater mechanical strength which can resist the shrinkage of the particle shells brought about the difference in density between the polymer and the monomer [35]. In addition, dimples can also be observed on the surface of all the PSMA/n-octadecane microPCMs particles, as shown in Fig. 2c, d. The similar appearances were coincided in different microPCMs prepared using acrylic resin as shell materials [24, 25, 27]. It seems to imply that this dimples due to the shrinkage of the microcapsules, which is caused by a production of reserved expansion space in the progress of the microPCMs fabrication. This reserved expansion resulted from the reduced volume of n-octadecane as its phase changes from melting state to solidification state and from the higher density of the polymer as compared with the monomers as explained in several studies [16, 27, 36]. Furthermore, it was found that the deformation of the PSMA/n-octadecane microPCM with PETA attributed to the shell shrinkage of the microcapsule was more serious than that of the PSMA/n-octadecane microPCM with DVB, showing a deflated ball morphology of the PSMA/n-octadecane microPCM with PETA. In addition, there were aggregations between all the PSMA/n-octadecane microPCMs particles. Nevertheless, the degree of adhesions of the PSMA/n-octadecane microPCM appeared to be improved, and the morphologies tend to be more regular, when PCM microencapsulated using DVB as the crosslinking agent. These results agree well with our earlier findings of the difference between the morphologies of PBMA/n-octadecane microcapsules with PETA and DVB [37], indicating that these facts are probably due to the rigid phenyl group connecting with the crosslinkable functional moieties of DVB [37]. The rigid phenyl group of DVB can lead to an increase rigid of the polymer chain by restricting atom in the polymer chain to rotate, so that a higher stiffness of the crosslinked PSMA shell with DVB as compared with the crosslinked PSMA shell with PETA. The great mechanical strength can better resist external pressure caused by the phase changes of PCM from melting state to solidification state and the centrifugation for separating after the process of encapsulation, thus resulting in a more regular morphology and a better dispersion of the microPCMs particle.
Figure 3 demonstrated the particle size distributions (PSD) of the microcapsules. As shown in Fig. 3, the PSD of both the PSMA/n-octadecane microPCM with PETA and the microPCM with DVB show unimodal distributions. However, their PSD are quite different from each other. The particle sizes of the PSMA/n-octadecane microPCM with PETA varied from 0.1 to 18 µm with average diameter of 5 µm, and more than 60 % of them had particle sizes of 2–6 µm. The particle sizes of the PSMA/n-octadecane microPCM with DVB ranged from 3 to 68 µm with mean diameter of 21 µm, and most of the PSMA/n-octadecane microPCM with DVB particles are below 20 µm. It is found that a more narrow size distribution with a lower mean diameter of the microPCM with PETA was obtained. This result is in consonance with our earlier findings of the difference between the PSD of PBMA/n-octadecane microcapsules with PETA and DVB [37]. Sánchez [38] assumed that the PSD will be influenced by the hydrophilic nature of the polymer. Accordingly, it seems to suggest that the relatively narrow PSD with a lower mean diameter possibly related to the hydrophilic nature of the PSMA shell with PETA brought about the presence of a carboxyl group in the PETA. The hydrophilic of the PSMA shell with PETA normally caused a greater stability of the polymer droplet in the suspension process [38, 39]. The increased stability of the polymer droplet can decrease the coalescence of the polymer droplets in the encapsulation process, leading to a smaller particle and a narrow PSD of the microPCM with PETA compared with the microPCM with DVB.
Thermal properties of microcapsules
The heating and cooling curves of pure n-octadecane and the as-prepared microPCMs are presented in Fig. 4. As shown in Fig. 4a, b, PSMA/n-octadecane microPCM with PETA melts at 22.5 °C, crystallizes at 21.9 °C as pure n-octadecane melts at 25.5 °C, and crystallizes at 23.7 °C. It can be seen from Fig. 4c, PSMA/n-octadecane microPCM with DVB melts at 21.8 °C and crystallizes at 21.5 °C. Although the melting points and crystallization points of both of the microPCMs with PETA and DVB decreased slightly as compared with those of the pure n-octadecane, it is noteworthy that the two kinds of microPCMs have desirable melting points in the range of 20.0 and 26.0 °C, which are close to human comfort temperature, to be applied in solar energy-saving buildings. Additionally, the phase change temperature span ΔT (ΔT = T em - T om) of both the PSMA/n-octadecane microPCM with PETA and with DVB were broadened by 3.6 and 6 °C, respectively, as compared with the pure n-octadecane. These results seem to suggest that the low thermal conductivity of the PSMA polymer, especially PSMA polymer shells fabricated using DVB as crosslinking agent, can slow down heat transferring through the microPCMs. Hence, the temperature range of melting enlarged, and the melting temperature decreased [40].
The phase change enthalpies of melting and freezing of PSMA/n-octadecane microPCM with PETA were measured to be 82.6 and 80.7 J g−1, respectively. These values for PSMA/n-octadecane microPCM with DVB were 87.9 and 94.8 J g−1, respectively. The n-octadecane content was calculated with the following formula as 36.3 % for PSMA/n-octadecane microPCM with PETA and 40.6 % for PSMA/n-octadecane microPCM with DVB, respectively.
where ΔH m,microPCM and ΔH c,microPCM are melting enthalpy and crystallization enthalpy of microPCMs, respectively; ΔH m, PCM and ΔH c, PCM are melting enthalpy and crystallization enthalpy of n-octadecane, respectively [17].
It is obvious that the phase change enthalpies and n-octadecane content of the PSMA/n-octadecane microPCM prepared using DVB were higher than that of the PSMA microPCM prepared using PETA. This is consistent with the result obtained from the surface morphologies of the as-prepared microPCM analysis. It seems to suggest that since SMA, the polymer shell forming monomer, has a long flexible side chain, introducing the rigid phenyl group of DVB can provide a greater mechanical strength of the PSMA shell. This enhanced mechanical strength of the polymer shell can probably lead to an increased PCM content of the microPCM, thus to a higher thermal storage capacity of the microPCM.
Thermal stabilities of microcapsules
The thermal stabilities of n-octadecane, PSMA polymer with DVB, and PSMA/n-octadecane microPCM with DVB were evaluated by means of TG, and their TG curves are shown in Fig. 5. n-Octadecane has one mass-loss step and started to lose its mass at approximately 165 °C attributed to the evaporation before boiling point (308 °C) or the decomposition at the flash point (166 °C) [17, 24]. Since n-octadecane is a long chain n-alkane with relatively lower decomposition temperature, the mass of pure n-octadecane dropped sharply to a low level with the temperature increasing. The PSMA polymer with DVB was degraded in one step at about 374 °C, which was accounted for a decomposition of the molecular chain. The TG curve of PSMA/n-octadecane microPCM with DVB has two mass-loss steps. The first mass-loss step started at about 238 °C. These are well correlated with earlier findings [16, 27, 36], indicating that the first mass-loss step corresponds to the leakage of the core PCM [16, 27, 36]. The second mass-loss step took place at the temperature of 376 °C, which was almost the same as the starting mass-loss temperature of the crosslinked PSMA polymer because of the decomposition of the PSMA shell. Apparently, the thermal resistant temperature of the PSMA/n-octadecane microPCM was significantly higher than that of the pure n-octadecane. Furthermore, the rate of the mass loss of the microencapsulated n-octadecane went down when compared with the pure n-octadecane. This result indicated that the PSMA shell can provide good protection for the core materials, and the leakage of the n-octadecane from the microcapsules can be prevented [41].
As can be seen from Fig. 5b, it is clear that the thermal resistance temperature of PSMA/n-octadecane microPCM with DVB was higher than that of PSMA/n-octadecane microPCM with PETA. This result is well correlated with the analysis of heat capacity, indicating that introducing rigid phenyl group of DVB may result in a higher mechanical strength of the PSMA shell and hence in a greater thermal stability of the PSMA/n-octadecane microPCM. In our precious work [27], it was found that the first mass-loss step of microcapsule containing n-octadecane with PMMA as shell using DVB as crosslinking agent started at about 226 °C. As shown in Fig. 5b, this value for PSMA/n-octadecane microcapsule using DVB as crosslinking agent was up to 238 °C. The greater thermal stability of PSMA/n-octadecane microPCM with DVB compared with PMMA/n-octadecane microPCM with DVB is a possible consequence of the greatest flexibility of SMA. The more flexible SMA, which is attributed to the significantly longer alkyl groups of SMA in contrast to MMA, can enhance the ability of the side chain to rearrange and form more ordered crystalline structures, hence resulting in a higher thermal resistance of the PSMA/n-octadecane microPCM [42].
Thermal reliabilities of microcapsules
Figure 6 shows the DSC curves of the PSMA/n-octadecane microcapsules after 1,000 thermal cycles. The melting points of the PSMA/n-octadecane microPCM with PETA and the PSMA/n-octadecane microPCM with DVB were slightly changed by 0.6 and 1.0 °C, respectively, when their crystallizing point moderately varied by 0.7 and 0.3 °C, respectively, after 1,000 thermal cycles. It can be worth noting that all the changes at phase change temperatures resulting from thermal cycles are very small. Moreover, after 1,000 thermal cycles, the measured phase change enthalpies of melting of PSMA/n-octadecane microPCM with PETA and DVB were 78.4 and 86.3 J g−1, respectively. And their phase change enthalpies of crystallization were measured as 79.7 and 85.8 J g−1, separately. It is evident that there were no significant changes in the phase change enthalpies of both PSMA/n-octadecane microPCM with PETA and PSMA/n-octadecane microPCM with DVB after the thermal cycling tests. These results indicated that the PSMA/n-octadecane microPCMs have good thermal reliabilities in term of their phase change temperatures and phase change enthalpies.
Temperature-regulated property of microcapsules
The surface temperature distributions of both the gypsum boards with and without PSMA/n-octadecane microPCM with temperature increasing were determined by means of infrared thermography. And the thermal images obtained from different times are presented in Fig. 7. It is obvious that the gypsum board with PSMA/n-octadecane microPCM endured a less notable heating effect than the gypsum board without microPCM. Thermoregulatory effect observed for the gypsum board containing PSMA/n-octadecane microPCM keeping at a constant temperature of 70 °C with respect to the gypsum board without microPCM was 2, 3, 4, and 5 °C after 93, 160, 228, 295, and 567 s, respectively. This result is consistent with earlier finding of thermoregulatory effect observed for the gypsum board containing P(BMA-co-MAA)/n-octadecane microcapsules keeping at a constant temperature of 60 °C [43]. It seems to suggest that the gypsum boards with incorporated PSMA/n-octadecane microPCM possessed temperature-regulated property keeping at a constant temperature of 70 °C, which is due to the amount of heat absorbed by n-octadecane in the melting process with the external temperature increasing. This thermoregulatory effect property of building materials can result in a reduced temperature fluctuation while maintaining desirable thermal comfort indoor.
Conclusions
Microcapsules containing n-octadecane with p(stearyl methacrylate) (PSMA) shells were prepared by a suspension-like polymerization. Pentaerythritol triacrylate and DVB were employed as the crosslinking agents. The microPCMs particles have a spherical profile with average diameter of 5 and 21 µm for PSMA/n-octadecane microPCM with PETA and PSMA/n-octadecane microPCM with DVB, respectively. The microPCM prepared using DVB exhibits a higher latent heats of melting (87.9 J g−1) and crystallization (94.8 J g−1) compared with the microPCM prepared using PETA. The thermal resistance temperatures of the as-prepared microcapsules were significantly increased as against the pure n-octadecane. PSMA/n-octadecane microPCM with DVB shows a greater thermal stability in comparison with PSMA/n-octadecane microPCM with PETA. The phase change temperatures and enthalpies of the as-prepared microcapsules varied little after thermal cycles. Therefore, microencapsulated n-octadecane with PSMA as shells, especially with PSMA shell prepared using DVB as crosslinking agent, has good thermal energy storage and thermal regulation potentials, such as solar energy-saving building materials.
References
Jegadheeswaran S, Pohekar S. Performance enhancement in latent heat thermal storage system: a review. Renew Sustain Energy Rev. 2009;13:2225–44.
Farid M, Khudhair A, Razack S, Hallaj S. A review on phase change energy storage: materials and applications. Energy Convers Manage. 2004;45:1597–615.
Jeon J, Lee J, Seo J, Jeong S, Kim S. Application of PCM thermal energy storage system to reduce building energy consumption. J Therm Anal Calorim. 2013;111:279–88.
Alkan C, Sari A, Karaipekli A, Uzun O. Preparation, characterization, and thermal properties of microencapsulated phase change material for thermal energy storage. Sol Energy Mater Sol Cells. 2009;93:143–7.
Chen Z, Yu F, Zeng X, Zhang Z. Preparation, characterization and thermal properties of nanocapsules containing phase change material n-dodecanol by miniemulsion polymerization with polymerizable emulsifier. Appl Energy. 2012;9:7–12.
Farid M, Khudhair A, Razack S, Al-Hallaj S. A review on phase change energy storage: materials and applications. Energy Convers Manag. 2004;45:1597–615.
Zhang Y, Zhou G, Lin K, Zhang Q, Di H. Application of latent heat thermal energy storage in buildings: state-of-the-art and outlook. Build Environ. 2007;42:2197–209.
Deveci S, Basal G. Preparation of PCM microcapsules by complex coacervation of silk fibroin and chitosan. Colloid Polym Sci. 2009;287:1455–67.
Basalm G, Deveci S, Yalcin D, Bayraktar O. Properties of n-eicosane-loaded silk fibroin-chitosan microcapsules. J Appl Polym Sci. 2011;121:1885–9.
Hawlader M, Uddin M, Zhu H. Preparation and evaluation of a novel solar storage material: microencapsulated paraffin. Int J Sol Energy. 2000;20:227–38.
Onder E, Sarier N, Cimena E. Encapsulation of phase change materials by complex coacervation to improve thermal performances of woven fabrics. Thermochim Acta. 2008;467:63–72.
Zhang X, Tao X, Yick K, Fan Y. Expansion dpace and thermal stability of microencapsulated n-octadecane. J Appl Polym Sci. 2005;97:390–6.
Jin Z, Wang Y, Liu J, Yang Z. Synthesis and properties of paraffin capsules as phase change materials. Polymer. 2008;49:2903–10.
Zhang X, Fan Y, Tao X, Yick K. Crystallization and prevention of supercooling of microencapsulated n-alkanes. J Colloid Interface Sci. 2005;281:299–306.
Sánchez L, Sánchez P, Lucas A, Carmona M, Rodríguez J. Microencapsulation of PCMs with a polystyrene shell. Colloid Polym Sci. 2007;285:1377–85.
Li W, Song G, Tang G, Chu X, Ma S, Liu C. Morphology structure and thermal stability of microencapsulated phase change material with polymer shell. Energy. 2011;36:785–91.
Su J, Wang L, Ren L, Huang Z, Meng X. Preparation and characterization of polyurethane microcapsules containing n-octadecane with styrene-maleic anhydride as a surfactant by interfacial polycondensation. J Appl Polym Sci. 2006;102:4996–5006.
Kim E, Do K. Preparation and properties of microencapsulated octadecane with waterborne polyurethane. J Appl Polym Sci. 2005;96:1596–604.
Alkan C, Sarı A, Karaipekli A. Preparation, thermal properties and thermal reliability of microencapsulated n-eicosane as novel phase change material for thermal energy storage. Energy Convers Manage. 2011;52:687–92.
Sarı A, Alkan C, Karaipekli A, Orhan U. Microencapsulated n-octacosane as phase change material for thermal energy storage. Sol Energy. 2009;83:1757–63.
Sarı A, Alkan C, Karaipekli A. Preparation, characterization and thermal properties of PMMA/n-heptadecane microcapsules as novel solid-liquid microPCM for thermal energy storage. Appl Energy. 2010;87:1529–34.
Chang C, Tsai Y, Chiu J, Chen H. Preparation of phase change materials microcapsule by using PMMA network-silica hybrid shell Via sol-gel process. J Appl Polym Sci. 2009;112:1850–7.
Ma S, Song G, Li W, Fan P, Tang G. UV irradiation-initiated MMA polymerization to prepare microcapsules containing phase change paraffin. Sol Energy Mater Sol Cells. 2010;94:1643–7.
Shan X, Wang J, Zhang X, Wang X. Formaldehyhe-free and thermal resistance MicroPCMs containing n-octadecane. Thermochim Acta. 2009;494:104–9.
Sánchez L, Tsavalas J, Sundberg D. Synthesis and characterization of paraffin wax microcapsules with acrylic-based polymer shells. Ind Eng Chem Res. 2010;49:12204–11.
Ma Y, Chu X, Li W, Tang G. Preparation and characterization of poly(methyl methacrylate-co-divinylbenzene) microcapsules containing phase change temperature adjustable binary core materials. Sol Energy. 2012;86:2056–66.
Qiu X, Li W, Song G, Chu X, Tang G. Fabrication and characterization of microencapsulated n-octadecane with different crosslinked methylmethacrylate-based polymer shells. Sol Energy Mater Sol Cells. 2012;98:283–93.
Yang R, Xu H, Zhang Y. Preparation, physical property and thermal physical property of phase change microcapsule slurry and phase change emulsion. Sol Energy Mate. SolarCells. 2003;80:405–16.
Alay S, Göde F, Alkan C. Synthesis and thermal properties of poly(n-butyl acrylate)/n-hexadecane microcapsules using different cross-linkers and their application to textile fabrics. J Appl Polym Sci. 2011;120:2821–9.
Meier M, Metzgerb J, Schubert U. Plant oil renewable resources as green alternatives in polymer science. Chem Soc Rev. 2007;36:1788–802.
Shang S, Huang S, Weiss R. Synthesis and characterization of itaconic anhydride and stearyl methacrylate copolymers. Polymer. 2009;50:3119–27.
Dutertre F, Pennarun P, Colombani O, Nicol E. Straightforward synthesis of poly(lauryl acrylate)-b-poly(stearyl acrylate) diblock copolymers by ATRP. Eur Polymer J. 2011;47:343–51.
Sánchez P, Sánchez M, Romero A, Rodríguez J, Sánchez L. Development of thermo-regulating textiles using paraffin wax microcapsules. Thermochim Acta. 2010;498:16–21.
Qiu X, Li W, Song G, Chu X, Tang GY. Microencapsulated n-octadecane with different methylmethacrylate-based copolymer shells as phase change materials for thermal energy storage. Energy. 2012;46:188–99.
Pascu O, Garcia R, Giamberini M. Interfacial polymerization of an epoxy resin and carboxylic acids for the synthesis of MicroPCMs. Polym Int. 2008;57:995–1006.
You M, Zhang X, Wang J, Wang X. Polyurethane foam containing microencapsulated phase-change materials with styrene-divinybenzene co-polymer shells. J Mater Sci. 2009;44:3141–7.
Qiu X, Song G, Chu X, Li X, Tang G. Microencapsulated n-alkane with p(n-butyl methacrylate-co-methacrylic acid) shell as phase change materials for thermal energy storage. Sol Energy. 2013;91:212–20.
Sánchez L, Sánchez P, Carmonam M, Lucas A, Rodríguez J. Influence of operation conditions on the microencapsulation of PCMs by means of suspension-like polymerization. Colloid Polym Sci. 2008;286:1019–27.
Zhang X, Fan Y, Tao X, Yick K. Fabrication and properties of microcapsules and nanocapsules containing n-octadecane. Mater Chem Phys. 2004;88:300–7.
Li W, Zhang X, Wang X, Niu J. Preparation and characterization of microencapsulated phase change material with low remnant formaldehyde content. Mater Chem Phys. 2007;106:437–42.
Li W, Wang J, Wang X, Wu S, Zhang X. Effects of ammonium chloride and heat treatment on residual formaldehyde contents of melamine-formaldehyde MicroPCMs. Colloid Polym Sci. 2007;285:1691–7.
Livshin S, Silverstein M. Cross-linker flexibility in porous crystalline polymers synthesized from long side-chain monomers through emulsion templating. Soft Matter. 2008;4:1630–8.
Qiu X, Song G, Chu X, Li X, Tang G. Preparation, thermal properties and thermal reliability of microencapsulated n-octadecane with acrylic-based polymer shells for thermal energy storage. Thermochim Acta. 2013;551:136–44.
Acknowledgements
The authors gratefully acknowledge the financial supports from Independent Research Project in Jiangnan University (No. JUSRP11453).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qiu, X., Lu, L., Zhang, Z. et al. Preparation, thermal property, and thermal stability of microencapsulated n-octadecane with poly(stearyl methacrylate) as shell. J Therm Anal Calorim 118, 1441–1449 (2014). https://doi.org/10.1007/s10973-014-4040-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-4040-8