Abstract
This study deals with fabrication, thermal properties and thermal reliabilities of microencapsulated paraffin by suspension-like copolymerization of ethyl methacrylate (EMA) with acrylic acid (AA) and styrene (St). Trimethylolpropanetriacrylate (TMPTA) was employed as crosslinking agents. The microencapsulated phase change materials (MicroPCMs) were characterized by using scanning electron microscopy (SEM), differential scanning calorimetry and thermal gravimetric analysis (TG). The microcapsule with P(EMA-co-AA-co-St-co-TMPTA) copolymer as shell exhibits higher phase change enthalpies of melting (117.8 J g−1) and crystallization (115.3 J g−1) compared with the microcapsule with P(EMA-co-AA-co-TMPTA) copolymer. TG analysis demonstrated that the thermal resistant temperatures of these two MicroPCMs were increased by more than 10 °C compared with the raw paraffin. The PCMs content of these two MicroPCMs dropped by <6 % after 1000 thermal cycling. Thermal images showed that the foam with microcapsule exhibits an upgraded thermal regulation capacity. As a consequence, microcapsules with EMA-based copolymer possess good potentials for thermal energy storage and thermal regulation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Latent heat storage is an attractive technique for a more effective use of renewable energy and residual heat. Phase change materials (PCMs) can generally be used as latent heat storage materials due to their large heat storage densities and their capacities of preserving a system in a tiny temperature variation via absorbing/releasing heat at a nearly isothermal process [1–4]. As a mixture of straight-chain n-alkanes, the fusion/crystallization of paraffin can absorb/release large amounts of latent heat. Besides, phase segregation and super-cooling rarely occur for this PCM due to its feature of congruent melting [5]. Microencapsulation is considered to be one of the most effective techniques for the integration of PCMs into matrix materials to provide them energy storage abilities [6]. With microencapsulation, the outside polymer shell can prohibit the interplay between PCMs and the matrix material, such as building materials, fiber and foam. Moreover, the smaller size of microencapsulation PCMs (MicroPCMs) can provide a greater surface/volume ratio, which can enhance the heat transfer rate [7]. Additionally, MicroPCMs can produce some more merits, such as avoiding the melted PCMs escaping from matrix material and reducing volume change rate of the PCMs during phase change process [2, 6]. These advantages enable MicroPCMs more safe, stable and effective with respect to conventional PCMs when used in heat storage and thermal control applications. For instance, the MicroPCMs can be incorporated into conventional heat-insulating foams to improve their thermoregulation capacities, which are widely applied in energy-saving construction materials, thermal regulation materials of clothing and automotive interiors and transport package of medical and food product [8–11].
Polymer shells play a key role in adjusting heat capabilities, thermal stabilities and thermal reliabilities of microcapsules. Choosing copolymer as shell material had aroused more and more attentions because the performance of the copolymer can be controlled by altering its compositions [12–15]. Ethyl methacrylate (EMA) is an acrylic monomer with commercial availability at a reasonable price. Yang et al. [16] utilized polystyrene(PSt), polymethyl methacrylate (PMMA) and polyethyl methacrylate (PEMA) as shell materials to synthesis microcapsules containing tetradecane. Their results show that the softest polymer shell of the three, PEMA, could be advantageous for microencapsulation of n-alkane. Sánchez et al. [13, 14] used methyl methacrylate (MMA), methyl acrylate(MA), methacrylic acid (MAA) and styrene (St) as shell-forming monomer to synthesis paraffin-containing MicroPCMs. They found that an increased polarity gap between monomers and paraffin could lead to a greater driving force for phase separation of the copolymer shell formed within the paraffin droplet. Xu et al. [15] chose acrylic acid (AA), a water solubility carboxylic acid with significantly higher water solubility compared with paraffin, to copolymerize with MAA for microencapsulation of paraffin. However, there is rare report on preparation of paraffin-containing microcapsules by copolymerization of EMA with AA. Moreover, though EMA-based polymers with lower glass transition temperature (Tg) have greater film-forming abilities as compared with MMA-based polymers, the films formed by them exhibit low Young’s moduli [17]. Styrene (St), a commercially available monomer with a rigid group, can provide a greater strength of copolymer shells. Nevertheless, PSt, which is formed by homopolymerization of St, is a brittle compound. Accordingly, St can be introduced as a hard monomer to copolymer with soft monomer to compromise these two aspect. Chen et al. [18] and Fang et al. [19] prepared phase change material nanocapsules with n-alkance as the core and St-butyl acrylate copolymer (BA) copolymer as the shell. Sánchez et al. [14] microencapsulated PCMs with a St-MMA copolymer shell. In addition, highly crosslinked micron-sized spherical polymer particles have received much attention due to their superior strength, thermal and solvent resistance [20]. Konuklu et al. [21] investigated microencapsulation PCMs in poly(ethylacrylate) (PEA) shells by using ethylene glycol dimethacrylate, a crosslinking agent with two crosslinkable functional moieties. Ha et al. [20] successfully synthesized crosslinked stable microspheres by using St and three crosslinking agents. These crosslinking agents are ethylene glycol dimethacrylate (EGDMA), trimethylolpropane trimethacrylate (TMPTMA) and pentaerythritol tetraacrylate (PETRA), in which there are two, three and four crosslinkable functional moieties, respectively. They found that the minimum concentrations for forming the stable spherical particles reduced and the TG onset point of the microsphere increased with the increase in the number of crosslinking functional moieties. Additionally, the yields of microsphere with EGDMA, TMPTMA PETRA were higher than that of microspheres with DVB. In our previous study [22], MicroPCMs with crosslinked MMA-based polymer were fabricated by using three crosslinking agents. They were 1, 4-butylene glycol diacrylate (BDDA) and divinylbenzene (DVB), which contain two crosslinkable functional moieties, and trimethylolpropanetriacrylate (TMPTA) with three crosslinkable functional moieties. It was found that the PCMs content of microcapsule with TMPTA increased compared with microcapsule with BDDA and DVB due to the higher crosslinking density. Consequently, the objective of this research was to synthesis MicroPCMs containing paraffin with ternary copolymer [P(EMA-co-AA-co-TMPTA)] and tetracopolymer [P(EMA-co-AA-co-St-co-TMPTA)] as shells. Subsequently, phase change properties, thermal stabilities and thermal reliabilities of MicroPCMs were investigated and compared. Additionally, the MicroPCMs with EMA-based copolymer were integrated into polystyrene foam (EPSF) to investigate their temperature-regulated properties.
Experimental
Materials
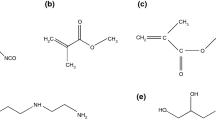
Ethyl methacrylate (EMA, C.R.) purchased from Sinopharm was utilized as shell-forming monomer. Acrylic acid (AA, C.R.) and styrene (St, C.R.) from Sinopharm were used to copolymerize with EMA. Trimethylolpropanetriacrylate (TMPTA, 80 mass%) obtained from Xinlimei Technology Ltd., was employed as a crosslinker. Paraffin (99 mass%) from Alfa was chosen as a core material. Sodium salt of styrene–maleic anhydride polymer purchased from Shanghai Leather Chemical Works was selected as a suspension stabilizer. 2,2-Azobisisobutyronitrile (AIBN, A.R.) from Sinopharm was chosen as an initiator. Styrene–butadiene latex (49–51 mass%) obtained from BASF was utilized as a binder.
Preparation of microcapsules
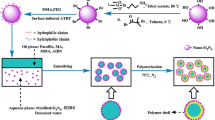
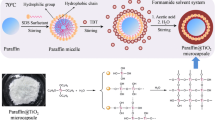
Microcapsules have been fabricated by a suspension-like polymerization technique. Two phases, an aqueous phase and an oil phase, were involved in the synthesis system. In the case of paraffin, microencapsulation, monomers, crosslinker, paraffin and oil-soluble initiator were assembled as oil phase. And water and a small amount of suspension dispersive agent formed aqueous phase, while the preparation of polymer particle without PCM was carried out through the same technique excepting the oil phase without paraffin. The two phases were mixed at a wile agitation of 1050 rpm at 50 °C for 15 min for a formation of a steady emulsion. Then, suitable polymerization temperature was determined as 85 °C. The polymerization process maintained for 6 h with a stirring speed of 520 rpm. Once the microcapsules were obtained, they were concentrated by centrifugation at 2000 rpm for 5 min. And then the MicroPCMs particles were purified by washing repeatedly in ethanol solution and filtered. The particles were dried for 72 h at 40 °C. The fabrication of MicroPCMs under the same condition was repeated three times. The dry weight of each sample versus different recipes was measured by electronic balance. Formula compositions of the samples and the dry weight of MicroPCMs are given in Table 1. As can be seen from Table 1, the yield of microcapsule with [P(EMA-co-AA-co-St-co-TMPTA)] was slightly lower than that of microcapsule with [P(EMA-co-AA-co-TMPTA)]. Ha et al. [20] found that the yields of microsphere with the copolymerization of St with acrylate monomers were higher than that of microspheres with the copolymerization of St with DVB. They assumed that this was due to that the residual vinyl groups connecting with flexible aliphatic unit of acrylate monomers on the gel and out layer of polymer particles were easier to react with the oligomer than the vinyl groups connecting with rigid phenyl group of DVB, to result in the growth of the polymer. Accordingly, in the present study, the residual vinyl group in St connects with rigid phenyl group, whereas the residual vinyl groups EMA and AA connect with flexible aliphatic unit which were more favorable to react with the oligomer to lead to a higher monomer conversion, hence to a higher yield with P(EMA-co-AA-co-TMPTA) than with P(EMA-co-AA-co-St-co-TMPTA).
Characterization of microcapsules
Surface features of EMA-based copolymer particles and MicroPCMs were investigated with a scanning electron microscope instrument (HITACHI S4800, Japan). Particle size distributions (PSD) of the MicroPCMs particles were obtained by using a Nano measurer 1.2.5 Program to measure from more than 300 MicroPCMs particles on the SEM micrographs.
Measurement of melting/crystallization temperatures and melting/crystallization enthalpies of the MicroPCMs and paraffin was carried out on a differential scanning calorimetry (DSC, TA, Q2000, USA). Eight milligrams of the microcapsules or paraffin was sealed in an aluminum crucible and then tested at a heating/cooling rate of 5 °C min−1 in a constant nitrogen stream of 60 mL min−1. The calculation formula for paraffin content in MicroPCMs was presented as following [23]:
where ΔH m,MicroPCMs and ΔH m,PCMs are measured melting enthalpies of MicroPCMs and PCMs itself, respectively; and ΔH c,MicroPCMs and ΔH c,PCMs are measured crystallization enthalpies of MicroPCMs and PCMs itself, respectively.
Thermal stabilities of EMA-based copolymer/paraffin microcapsule and paraffin were determined by using a thermal gravimetric analysis (TG, TA, Q500, USA). The analyses were conducted with a heating rate of 10 °C min−1 in a temperature range of 40–600 °C under a constant nitrogen stream of 50 mL min−1.
The cooling–heating cycle was carried out by using a closed thermal cycling experiment box (BPH-060B, Shanghai Yiheng Scientific Instruments Equipment Co., Ltd, China) according to literature [24]. The temperature and latent heat variation after cooling–heating cycle of 500 times were evaluated by the same DSC instrument
A temperature ascending experiment was carried out to investigate thermal control performances of foam with EMA-based copolymer/paraffin microcapsule [25, 26]. MicroPCMs were integrated to EPSF based on a surface coating technique. The raw foam was immersed into adhesive solution having 50 gL−1 microcapsules dispersed therein. This process was allowed to maintain for 1 h. Then, the foam sample was put into oven for 30 min with a constant temperature of 60 °C. Foam-a contained P(EMA-co-AA-co-St-co-TMPTA)/paraffin microcapsule, and Foam-b without microcapsule was used as a reference. Both of these foams were kept in a refrigerator set at 4 °C for 1 h and then placed in a beaker immersed into 40 °C thermostatic bath. Surface temperatures of foams with and without microcapsulates at various moments with increased temperatures were analyzed by using an infrared thermography (FLIR, T400).
Results and discussion
Morphologies and particle size distributions of microcapsules
The surface features of the microcapsule particles obtained in each case were investigated with SEM, as shown in Fig. 1. MicroPCMs with P(EMA-co-AA-co-TMPTA) as shell have spherical profiles. However, MicroPCMs with P(EMA-co-AA-co-St-co-TMPTA) possessed a rod-like shape besides the spherical shape, showing an inhomogeneous morphology. A similar appearance was observed in other MicroPCMs whose macromolecular compositions contained St [27, 28]. This fact could be related to a decreased polarity of St. Water solubility of St is 0.5 gL−1 (20 °C), which is about one-eighth of that of EMA (4 gL−1, 20 °C). And the polarity of St is more similar to paraffin, which may lead to a less thermodynamically favored reaction system, thus to a more irregular morphology of the microcapsule [13]. Additionally, it is apparent that MicroPCMs particles with P(EMA-co-AA-co-TMPTA) as shell were severely agglomerated. Nevertheless, the reunite of the MicroPCMs particles got obviously improvement by introducing St to the copolymer shells. The result was in accordance with the finding of Ma et al. [29]. They prepared MicroPCMs with P(BA-co-MMA-co-DVB) and P(BA-co-St-co-DVB) and found that MicroPCMs showed better disperse ability when MMA was replaced by St. Ha et al. [20] and Shan et al. [12] investigated the influence of the degree of crosslinking on the morphology of microcapsule with St-based shell and MMA-based shell. They assumed that the high degree of crosslinking within the polymer prevents from coagulation between the particles due to the improved hardness and the elastic modulus. Accordingly, this fact could be explained considering the change of mechanical strength when St was introduced to the polymer shell. The incorporated rigid group of St can increase stiffness of polymer chain by limiting the movement of molecular chain, leading to an increase hardness and strength of the shell to prevent the particle aggregation in course of the polymerization or during post-treatment. Hence, an improved dispersity of MicroPCMs particles with P(EMA-co-AA-co-St-co-TMPTA) was obtained.
Figure 2 shows particle size distributions (PSD) of microcapsules. Figure 3 shows that the PSD of these particles differed from one another. Paraffin/P(EMA-co-AA-co-TMPTA) microcapsule showed unimodal PSD, while microcapsules with P(EMA-co-AA-co-St-co-TMPTA) as shell showed multimodal PSD. The particle diameters of the microcapsules with ternary copolymer shell and tetracopolymer shell have size distribution in a range from 0.4 to 50 µm and from 0.7 to 64 µm, respectively. The mean particle size was 7 µm for the former and 21 µm for the latter. Apparently, sizes of microcapsules increased, and PSD was wider with the presence of St in the copolymer shell. One factor that may be contributing toward the higher diameter and wider size distribution is the poorer stability of monomer–paraffin mixture droplet when St was introduced to the copolymer shell [13]. Another probable factor is an increased viscosity of the monomer–paraffin mixture droplet resulting from the incorporation of St with higher viscosity (0.78 m Pa s, 20 °C) as compared with EMA (0.63 m Pa s, 20 °C), which can cause the monomer droplet more difficult to break into a smaller one [30]. Both two factors may lead to a greater aggregation of monomer–paraffin mixture droplet in the encapsulation process, hence to an increased diameter with a relatively wider PSD of microcapsules with P(EMA-co-AA-co-St-co-TMPTA) as shell.
Phase change properties of microcapsules
DSC curves of pure paraffin as well as microcapsules using bipolymer and ternary polymer as shells are given in Fig. 3a, b. There are two phase change peaks on both cooling curve and heating curve of paraffin and paraffin-containing microcapsules. Some researchers pointed out that paraffin, a mixture of n-alkane, can probably experience multi-step phase transition since several stable and partially ordered phases exist in some of the n-alkane [3, 31, 32]. Accordingly, the minor peak at the lower temperature represents solid–solid phase transition, the order–disorder transitions in PCMs. The main peak corresponds to solid–liquid phase change, the melting/fusing process of paraffin. It is clear that the melting peak temperature of both microcapsules with ternary and tetracopolymer as shell increased by approximately 1.5 °C and the crystallization peak temperature of these microcapsules decreased by around 2.5 °C as compared with pure paraffin. This suggests that EMA-based copolymer shell with low thermal conductivity, which covered on paraffin, would hinder phase change transferring from passing through MicroPCMs [33, 34].
The phase change enthalpies and PCM content of microcapsules are the critical factor in microencapsulation of PCM. It is in direct relation to thermal storage capability and thermal regulation of MicroPCMs [35]. The phase change enthalpies and phase change temperature corresponding to solid–liquid transition (melting and freezing) of the microcapsules and the content of PCMs in the microcapsules are given in Table 2. Figure 4b and Table 2 show that both MicroPCMs with ternary copolymer and tetracopolymer have more than 30 % higher melting enthalpies than PEMA-encapsulated tetradecane slurry (80.6 J g−1) by Yang et al. [16] and slightly higher than that (101 J g−1) of paraffin/PMMA microcapsule synthesized by Ma et al. [25]. Moreover, it was found that the PCMs content of all the as-prepared microcapsules was higher than that (25.7–48.8 mass%) of caprylic acid/PEA microcapsule crosslinked with EGDMA containing two crosslinkable functional moieties by Konuklu et al. [21], and that (27.7–50.7 %) of n-hexadecane/PBA microcapsules crosslinked with three crosslinking, which also contain two crosslinkable functional moieties by Alay et al. [36]. These results suggest that the phase change property of microencapsulated paraffin can be satisfactorily applied in thermal energy storage and thermal regulation. Nevertheless, it is evident that paraffin content was marginally increased by about 5 mass% when St introducing to the copolymer shell. Paraffin/P(EMA-co-AA-co-St-co-TMPTA) microcapsule has the higher phase change enthalpies of melting (117.8 J g−1) and crystallization (115.3 J g−1) accompanying with the higher paraffin content (70.8 mass%) in comparison with paraffin/P(EMA-co-AA-co-TMPTA) microcapsule. Sánchez et al. [14] microencapsulated paraffin by a copolymerization of St and MMA. The result shows that when an MMA/St proportion rose to 5.0 the polymer tended to polymerize outside the bead in a segregated way due to the hydrophilicity of MMA monomer. Zhou et al. [37] and Jang et al. [38] prepared St-based and 4-tert-butylstyrene (tBS)-based copolymer for oil absorbency. They assumed that the St and tBs monomer with phenyl group may have some stereo effect to produce the crosslinked polymer with a large cavity in which oil will fill. Accordingly, the increase in paraffin content in present study may be due to the hydrophobicity and stereo effect of the St, which could lead to a better paraffin absorbing and paraffin holding, thus to a higher paraffin content of MicroPCMs with P(EMA-co-AA-co-St-co-TMPTA).
Thermal stabilities of microcapsules
TG curves of microencapsulated paraffin and paraffin itself are given in Fig. 4. The mass reduction of paraffin began at around 146 °C and came to an end at roughly 207 °C. These mass-loss stages may be attributed to the evaporation or the decomposition of paraffin [12, 39]. The thermal degradation of EMA-based copolymer has one stage with the initial mass-loss temperature of roughly 366 °C for ternary copolymer and 379 °C for tetracopolymer, which is due to the decomposition of macromolecular chains. It is obvious that TG curves of microcapsules with ternary copolymer and tetracopolymer have a similar profile, and both of them degraded in two stages. The first stage started at approximately 156 °C for microcapsule with ternary copolymer and 164 °C for microcapsule with tetracopolymer, respectively. These are in agreement with the observation made by other researcher [12, 39–41]. This suggests that the first mass-loss step is probably a consequence of the diffusion of n-alkane out of polymer shell, which may be evaporate or decompose as temperature rose. It can also be found that the second thermal degradation of both microcapsules with ternary copolymer and tetracopolymer started at much the same initial mass-loss temperature as EMA-based copolymer. The result indicates that this degradation stage resulted from the decomposition of shell material. Additionally, it is evident that the initial mass-loss temperature of both microencapsulated paraffin was remarkably increased by over 10 °C as compared with pure PCM. This suggests that EMA-based copolymer shell could prevent paraffin in MicroPCMs from rapid mass loss [42, 43]. In addition, it can be found that the resistant temperature of MicroPCMs with P(EMA-co-AA-co-St-co-TMPTA) as shells was increased by 8 °C in comparison with the MicroPCMs with P(EMA-co-AA-co-TMPTA) as shells. This result is consistent with the finding of Ma et al. [29]; the onset decomposition temperature of MicroPCMs with P(BA-co-St-co-DVB) was higher by 9.4 °C than that of MicroPCMs with P(BA-co-MMA-co-DVB), indicating that thermal resistant temperature is also in relation to the introduction of benzene ring to the copolymer shell. The rigid ring can constrict atom of the macromolecular chain to rotate to increase the rigidity of the polymer chain shell, which could lead to an enhanced thermal resistant temperature of MicroPCMs with P(EMA-co-AA-co-St-co-TMPTA) shell.
Thermal reliabilities of microcapsules
Figure 3c shows DSC curves of the microcapsule with P(EMA-co-AA-co-TMPTA) and P(EMA-co-AA-co-St-co-TMPTA) as shells which experienced thermal cycles. The melting points of the microcapsules with ternary and tetracopolymer were slightly changed by 0.9 and 0.1 °C, respectively, when their crystallizing point moderately varied by 0.2 and 0.1 °C, respectively, after thermal cycles. Apparently, all the variations in the phase change temperatures of the EMA-based microcapsules resulting from 1000 thermal cycles are trivial. Furthermore, after thermal cycling, the reduction rate in the melting enthalpies and crystallization enthalpies of EMA-based microcapsules was lower than 15 %. Meanwhile, the PCMs content of these two MicroPCMs dropped by <6 % after thermal cycling. It is evident that there were no notable changes in the phase change enthalpies of both MicroPCMs with ternary and tetracopolymer shell after thermal cycling tests. However, the declines of PCM content of the as-prepared MicroPCMs were slightly higher than those of MicroPCMs with butyl methacrylate (BMA)-based, BA-based or stearyl methacrylate-based (SMA) polymer as shell by using pentaerythritol triacrylate (PETA) which also has three crosslinkable functional moieties in our previous study [32, 44]. This is probable attributed to an increased toughness of the BMA-based, BA-based or SMA-based (SMA) polymer shells brought about by longer flexible side chains as compared with the EMA-based polymer shell, which can better resist internal pressure in the process of thermal cycle.
Temperature-regulated property of microcapsule
Surface temperature distribution images of foam with microcapsule prepared by P(EMA-co-AA-co-St-co-TMPTA) as shell and untreated foam are shown in Fig. 5. The timer was started when MicroPCMs-treated foam and untreated foam were taken out of the refrigerator. Meanwhile, the surface temperatures of these two foams were measured by infrared thermography and shown in Fig. 5a. Then they were placed in a thermostatic bath, and the measurement of their surface temperature was continued. Obviously, the measured temperatures of the surface center of infrared thermography were almost the same at the beginning (Fig. 5a). As can be seen from Fig. 5, it can also be observed that MicroPCMs-treated foams had a less distinct thermo-affected experience than the untreated foam. Thermoregulatory influence on the MicroPCMs-treated foam with respect to the reference foam was 10.5, 9.3, 6.7 °C after 410, 712 and 807 s, respectively. The result indicates that the thermoregulation capacity of MicroPCMs-treated foam was improved attributed to a given ability of heat storage in the melting process of paraffin as temperature increased. The upgraded thermoregulation capacity can result in a sustainable improvement in human thermal comfort when the MicroPCMs-treated foams introduced into energy-saving construction materials and thermal regulation materials of clothing and automotive interiors.
Conclusions
Microencapsulation of paraffin was performed by a suspension-like copolymerization of ethyl methacrylate (EMA) with styrene (St) and acrylic acid (AA). Trimethylolpropanetriacrylate (TMPTA) was employed as crosslinking agents. SEM photographs indicated that MicroPCMs with P(EMA-co-AA-co-TMPTA) as shell have spherical profiles, while MicroPCMs with P(EMA-co-AA-co-St-co-TMPTA) possessed a rod-like shape besides the spherical shape. The diameter of them was about 0–65 µm. The microcapsule with P(EMA-co-AA-co-St-co-TMPTA) copolymer as shell exhibits higher phase change enthalpies of melting (117.8 J g−1) and crystallization (115.3 J g−1) compared with the microcapsule with P(EMA-co-AA-co-TMPTA) copolymer. In addition, TG investigation showed that the initial mass-loss temperature of MicroPCMs with ternary copolymer and tetracopolymer was remarkably increased by 10 and 18 °C, respectively, as compared with paraffin. Moreover, the melting enthalpies of these two MicroPCMs dropped by <15 %, and the paraffin content of them dropped by <6 % after 1000 thermal cycling. Thermal images showed that the foam treated by microcapsule with ternary shell shows an upgraded thermal regulation capacity. As a consequence, microcapsules with EMA-based copolymer shells possess good potentials for thermal energy storage and thermal regulation.
References
Regin A, Solanki S, Saini J. Heat transfer characteristics of thermal energy storage system using PCM capsules: a review. Renew Sustain Energy Rev. 2008;41:2438–58.
Jegadheeswaran S, Pohekar S. Performance enhancement in latent heat thermal storage system: a review. Renew Sustain Energy Rev. 2009;13:2225–44.
Jeon J, Lee J, Seo J, Jeong S, Kim S. Application of PCM thermal energy storage system to reduce building energy consumption. J Therm Anal Calorim. 2013;111:279–88.
Anghel E, Georgiev A, Petrescu S, Popov R, Constantinescu M. Thermo-physical characterization of some paraffins used as phase change materials for thermal energy storage. J Therm Anal Calorim. 2014;117:557–66.
Kenisarin M. Thermophysical properties of some organic phase change materials for latent heat storage. Rev Sol Energy. 2014;107:553–75.
Farid M, Khudhair A, Razack S. A review on phase change energy storage: materials and applications. Energy Convers Manag. 2004;45:1597–615.
Sharma A, Tyagi V, Chen C, Buddhi D. Review on thermal energy storage with phase change materials and applications. Renew Sustain Energy Rev. 2009;13:318–45.
Nihal S, Emel O. Thermal characteristics of polyurethane foams incorporated with phase change materials. Thermochim Acta. 2007;454:90–8.
You M, Zhang X, Wang J, Wang X. Polyurethane foam containing microencapsulated phase-change materials with styrene–divinybenzene co-polymer shells. J Mater Sci. 2009;44:3141–7.
Borreguero A, Rodríguez J, Valverde J, Arevalo R, Peijs T. Manuel Carmona Characterization of rigid polyurethane foams containing microencapsulated Rubitherm_RT27: catalyst effect. Part II. J Mater Sci. 2011;46:347–56.
Castellón C, Medrano M, Roca J, Cabeza L, Navarro M, Fernández A, Lázaro A, Zalba B. Effect of microencapsulated phase change material in sandwich panels. Renew Energy. 2010;35:2370–4.
Shan X, Wang J, Zhang X, Wang X. Formaldehyhe-free and thermal resistant MicroPCMs containing n-octadecane. Thermochim Acta. 2009;494:104–9.
Sánchez L, Tsavalas J, Sundberg D, Sánchez P, Rodriguez J. Synthesis and characterization of paraffin wax microcapsules with acrylic-based polymer Shells. Ind Eng Chem Res. 2010;49:12204–11.
Sánchez L, Rodriguez J, Romero A, Borreguero A, Carmona M, Sánchez P. Microencapsulation of PCMs with a styrene-methyl methacrylate copolymer shell by suspension-like polymerization. Chem Eng J. 2010;157:216–22.
Xu J, Wan X, Zhang B, Wang Y, Guo B, Zhang Y, Wang X. Wax/P(MMA-co-AA) core-shell microcapsules. Chin J Acta Polym Sin. 2009;11:1154–6.
Yang R, Xu H, Zhang Y. Preparation, physical property and thermal physical property of phase change microcapsule slurry and phase change emulsion. Sol Energy Mater Sol Cells. 2003;80:405–16.
Ai Z, Zhou Q, Xie C, Zhang H. In situ preparation and properties of high-solid-content and low-viscosity poly(methyl methacrylate/n-butyl acrylate/acrylic acid)/poly(styrene/acrylic acid) composite latexes. J Appl Polymer Sci. 2007;103:1815–25.
Chen C, Chen Z, Zeng X, Fang X, Zhang Z. Fabrication and characterization of nanocapsules containing n-dodecanol by miniemulsion polymerization using interfacial redox initiation. Colloid Polym Sci. 2012;290:307–14.
Fang Y, Kuang S, Gao X, Zhang Z. Preparation and characterization of novel nanoencapsulated phase change materials. Energy Convers Manag. 2008;49:3704–7.
Ha M, Lee K, Choe S. Crosslinkable functional moiety for the formation of highly crosslinked stable microspheres in the precipitation polymerization. Polymer. 2008;49:4592–601.
Konuklu Y. Microencapsulation of phase change material with poly (ethylacrylate) shell for thermal energy storage. Int J Energy Res. 2014;38:2019–29.
Qiu X, Li W, Song GL, Chu XG, Tang GY. Fabrication and characterization of microencapsulated n-octadecane with different crosslinked methylmethacrylate-based polymer shells. Sol Energy Mater Sol Cells. 2012;98:283–93.
Su J, Wang L, Ren L, Huang Z, Meng X. Preparation and characterization of polyurethane microPCMs containing n-octadecane with styrene-maleic anhydride as a surfactant by interfacial polycondensation. J Appl Polymer Sci. 2006;102:4996–5006.
Ma Y, Chu X, Li W, Tang G. Preparation and characterization of poly(methyl methacrylate-co-divinylbenzene) microcapsules containing phase change temperature adjustable binary core materials. Sol Energy. 2012;86:2056–66.
Ma S, Song G, Li W, Fan P, Tang G. UV irradiation-initiated MMA polymerization to prepare microcapsules containing phase change paraffin. Sol Energy Mater Sol Cells. 2010;94:1643–7.
Sánchez P, Sánchez M, Romero A, Rodríguez J, Sánchez L. Development of thermo-regulating textiles using paraffin wax microcapsules. Thermochim Acta. 2010;498:16–21.
Sánchez L, Sánchez P, Carmonam M, Lucas A, Rodríguez J. Influence of operation conditions on the microencapsulation of PCMs by means of suspension-like polymerization. Colloid Polym Sci. 2008;286:1019–27.
Sánchez L, Sánchez P, Lucas A, Carmona M, Rodríguez J. Microencapsulation of PCMs with a polystyrene shell. Colloid Polym Sci. 2007;285:1377–85.
Ma Y, Sun S, Li J, Tang G. Preparation and thermal reliabilities of microencapsulated phase change materials with binary cores and acrylate-based polymer shells. Thermochim Acta. 2014;588:38–46.
Song J, Chen L, Li X. Microencapsulation Technique and Application. Beijing: Chemical Industry Press; 2001. p. 74–5.
Sana S, Magali F, Igor K, Laurent I, Boumediene B, Yves C. Thermal characterization of polymer matrix composites containing microencapsulated paraffin in solid or liquid state. Energy Convers Manag. 2014;78:796–804.
Sullivan P. Solid-phase behavior of several long-chain n-paraffins, esters, and a ketone. J Res NBS A Phys Ch. 1974;78A(2):129–41.
Qiu X, Lu L, Zhang Z, Tang G, Song G. Preparation, thermal property, and thermal stability of microencapsulated n-octadecane with poly(stearyl methacrylate) as shell. J Therm Anal Calorim. 2014;118:1441–9.
Song G, Ma S, Tang G, Yin Z, Wang X. Preparation and characterization of flame retardant form-stable phase change materials composed by EPDM, paraffin and nano magnesium hydroxide. Energy. 2010;35:2179–83.
Alkan C, Sari A, Karaipekli A. Preparation, thermal properties and thermal reliability of microencapsulated n-eicosane as novel phase change material for thermal energy storage. Energy Conversat Manag. 2011;52:687–92.
Alay S, Alkan C, Göde F. Synthesis and characterization of poly(methyl methacrylate)/n-hexadecane microcapsules using different cross-linkers and their application to some fabrics. Thermochim Acta. 2011;518:1–8.
Zhou M, Cho W. Synthesis and properties of high oil-absorbent 4-tert-butylstyrene-EPDM-divinylbenzene graft terpolymer. J Appl Polym Sci. 2002;85:2119–29.
Jang J, Kim B. Studies of crosslinked styrene–alkyl acrylate copolymers for oil absorbency application. I. Synthesis and characterization. J Appl Polym Sci. 2000;77:903–13.
Li W, Song G, Tang G, Chu X, Ma S, Liu C. Morphology structure and thermal stability of microencapsulated phase change material with polymer shell. Energy. 2011;36:785–91.
Zhang H, Wang X, Wu D. Silica encapsulation of n-octadecane via sol–gel process: a novel microencapsulated phase-change material with enhanced thermal conductivity and performance. J Colloid Interface Sci. 2010;343:246–55.
Zhang X, Tao X, Yick K, Wang X. Structure and thermal stability of microencapsulated phase-change materials. Colloid Polym Sci. 2004;282:330–6.
Su J, Wang L, Ren L. Fabrication and thermal properties of MicroPCM: used Melamine-Formaldehyde resin as shell material. J Appl Polym Sci. 2006;101:1522–8.
Li W, Wang J, Wang X, Wu S, Zhang X. Effects of ammonium chloride and heat treatment on residual formaldehyde contents of melamine-formaldehyde MicroPCM. Colloid Polym Sci. 2007;285:1691–7.
Qiu X, Song G, Chu X, Li X, Tang G. Preparation, thermal properties and thermal reliability of microencapsulated n-octadecane with acrylic-based polymer shells for thermal energy storage. Thermochim Acta. 2013;551:136–44.
Funding
The authors would like to acknowledge the financial supported by the Fundamental Research Funds for the Central Universities (Nos. JUSRP51403A and JUSRP51511) and National Nature Science Foundation of China (No. 51503084).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qiu, X., Lu, L., Han, P. et al. Fabrication, thermal property and thermal reliability of microencapsulated paraffin with ethyl methacrylate-based copolymer shell. J Therm Anal Calorim 124, 1291–1299 (2016). https://doi.org/10.1007/s10973-016-5300-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5300-6