Abstract
Microcapsules containing PRS® paraffin wax (core) and a polystyrene shell were prepared by suspension-like polymerization. The influence of reaction temperature, stirring rate, and mass ratio of paraffin wax to styrene on the properties of phase change materials microcapsules was studied. The reaction temperature had not a significant effect on the size of the microcapsules but an increase of molecular weight and a narrow molecular weight distribution of polystyrene shell were observed when reaction temperature was increased. An exponential relationship between the stirring rate and the mean particle diameter in number has been found. It was observed that paraffin is difficultly encapsulated when the paraffin/polymer mass ratio was higher than 2.00, as a consequence of a shortage of polymer that could not completely cover the amount of paraffin added. However, when a large proportion of monomer was employed, the polymer tended to polymerize inside the droplets during the microencapsulation process forming complex inner structures. The microcapsules obtained have an interesting energy storage capacity of 153.5 J/g that makes them suitable for different applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microencapsulation is a very important technique in the industrial, agricultural, and medical fields by which small droplets of liquid or solid particles are coated with a shell. There are many examples of substances that have been encapsulated such as paints, liquid inks, toner, perfumes, pesticides, drugs, or phase change materials (PCMs) [1].
PCMs are materials with high heats of fusion. They can absorb or release the latent heat when the temperature of the material overpasses or undergoes the temperature of phase change. Much research has been conducted—and is indeed ongoing—with the aim to exploit this ability as energy reservoirs [2]. The final application depends directly on the kind of material used as PCM (melting point) as well as on the amount of PCM encapsulated.
Paraffin waxes compared to other PCMs have high heat-storage capacities, are easily available, and not expensive [3]. Moreover, a wide phase change temperature range within paraffins could be used depending on their chemical composition.
The PCM material confinement by microencapsulation facilitates their incorporation into a wide variety of applications such as in fibers, fabrics, coatings, physiotherapy devices, insulation panels, and walls [4]. In these applications, the PCM microcapsules are required to have good thermal characteristics and mechanical strength for ensuring intact structure during final use and manufacturing processes [5]. Therefore, the search of a physical and chemical appropriate shell is one of the main technical and scientific challenges on the encapsulation.
In recent years, a wide range of techniques has been developed to make PCM microcapsules, such as spray-drying, spray-cooling, emulsification, interfacial polycondensation, coacervation, or in situ polymerization [6–8].
In a previous work, phase change materials were successfully encapsulated by a polymer cover (polystyrene) by means of suspension-like polymerization technique [9]. The developed method was shown to be easy, cheap, and robust for the encapsulation of paraffin for textile and similar applications.
The efficiency and duration of the thermoregulatory effect, the active barrier effect in the surrounding substrate, and the thermo insulation effect in the fabric are conditioned by the particle size, the thermal capacity of the PCM, phase change temperature and structure, and type of the carrier as well as the substrate in which the microcapsules are included. Obviously, these microcapsule properties are affected by the synthesis conditions.
Independent of the encapsulation method employed, the main research efforts have been devoted to enlarge the thermal storage of the particles that depend directly on the amount of phase change material encapsulated [3, 7, 10]. Shin et al. [7] obtained 53 wt.% of phase change material encapsulated using coacervation method. Su et al. [8] reported the encapsulation of paraffin around 70 wt.% by in situ polymerization method. However, with reference to pure paraffin as PCM, the percentage of paraffin encapsulated was not more than 20 wt.% when paraffin is microencapsulated directly [11, 12].
For use in textile materials, an appropriate particle size should range from 0.5 to 100 μm [7]. Keeping in mind that the most important issue in the practical operation of suspension polymerization is the control of the final particle size distribution (PSD), the influence of the stirring rate, the reaction temperature, and the mass ratio of core to shell on properties of microcapsules containing PRS® paraffin wax with a polystyrene shell have been studied.
Materials and methods
Materials
Styrene (99 wt.%) was of reagent grade (Panreac Chemical). Styrene was washed with sodium hydroxide to remove the inhibitor and calcium chloride as desiccant. Benzoyl peroxide (BPO) (97 wt.%) was used as initiator (Fluka Chemical). PRS® paraffin wax (Mw 478 g/mol) was of commercial grade (Repsol YPF) and was used as core material. Polyvinylpyrrolidone (K30, Mw 40,000 g/mol) of reagent grade (Fluka Chemical) was used as stabilizer and methanol to pour the samples. All these reagents were used as received. Water was purified by distillation followed by deionization using ion-exchange resins. Nitrogen was of high purity grade (99.999%).
Microcapsules synthesis
PCM microcapsules containing PRS® paraffin wax were prepared by suspension-like polymerization technique. This process generally involves the dispersion of the monomer, mainly as a liquid in small droplets, into an agitated stabilizing medium usually consisting of water containing small amounts of suspension and dispersion agents, which was described in detail in [9]. In the case of PCM microencapsulation, the two phases (polymer and phase change material) may fully separate during the process and lead to core–shell morphology as “equilibrium” morphology, maintaining the phase change material inside the polymer covering layer that is being formed.
Three sets of different experiments were carried out by using different stirring rates, reaction temperatures, and mass ratios of PRS® paraffin wax/styrene. Figure 1 shows details of the experimental setup used.
Environmental scan electron microscopy observation
The surface features of the microcapsules and their diameters after polymerization were observed by using a XL30 (LFD) microscope for environmental scan electron microscopy.
Gel permeation chromatography measurement
The molecular weight distribution (MWD) of the polymer forming the shell and the relative amount of paraffin wax encapsulated were measured by gel permeation chromatography (GPC) using a chromatograph model 150-GPCV LC of Waters (USA). Tetrahydrofuran (THF) was used as elution solvent. Poly(styrene) standards from Waters were used for MWD calibration.
Calculation of number average diameter and volume average diameter
Particle size and PSD were determined on a Malvern Mastersizer Hydro 2000 SM light-scattering apparatus in a diluted dispersion of the particles in methanol.
Differential scanning calorimetry
Measurements of melting point and melting heat of pure paraffin wax and the microcapsules obtained were performed in a differential scanning calorimeter (DSC) model DSC Q100 of TA Instruments. These measurements were done varying the temperature in the range from −30 °C to 80 °C with a heating rate of 10 °C/min under a nitrogen atmosphere. The content of paraffin wax in the microcapsule can be estimated according to the measured melting enthalpy of different standard mixtures of polystyrene and paraffin wax. The experimental values obtained were fitted to a straight line with the following form:
where DeltaHm is the enthalpy of analyzed microcapsules (J/g).
Results and discussion
Influence of reaction temperature
The effect of polymerization temperature on the particle size and its morphology was investigated. Table 1 reports the thermal properties, the morphology, and the average diameter in volume (dpv0.5) and number (dpn0.5) of the microparticles obtained. When the reaction temperature was 78 °C, both volume average and number average diameter were lower than 1 μm. This fact indicates that the microencapsulation was not successful. It seems clear that the reaction temperature must be higher than the self-accelerating decomposition temperature of the initiator that has been established at 80 °C by the supplier; otherwise, the microencapsulation does not take place. There are probably radicals below that value but not enough to initiate properly the polymerization in this case.
Likewise, it can be observed in Fig. 3 that, above the activation temperature of initiator, the unimodal distribution of PSD and the volume average diameters remained unchanging (approximately 200 μm). As expected, our system exhibits the typical behavior of a suspension polymerization where the reaction temperature barely affects the time needed to reach the identity point. Hence, the PSD distribution will be more influenced by the type of stirring, the addition of surfactants, or the hydrophilic nature of the polymer than by the temperature [13].
On the other hand, when the reaction temperature was 128 °C, the microcapsules obtained did not have the typical homogeneous spherical shape (see Fig. 2) and showed a bimodal distribution with larger microcapsules (Fig. 3). This fact could be explained considering the shorter half-life of BPO at higher temperatures that leads to a lower efficiency in the initiation of polymerization, resulting in a greater instability of the encapsulation process that is reflected in irregular particle morphology or even can be considered as a case of dead-end polymerization as described by Odian [14].
Figure 4 shows GPC chromatograms of microcapsules obtained at different reaction temperatures. All of GPC analyses of microcapsules present two peaks: one at low elution time corresponding to the polystyrene cover with higher molecular weight and the other one with 478 g/mol corresponding to the low-weight product: PRS® paraffin wax. As the reaction temperature increases, the percentage of the integrated area corresponding to PRS® paraffin wax peak changes from 18% at 98 °C to 7% at 113 °C. This indicates that, as the polymerization temperature increases, the amount of encapsulated PRS® paraffin decreases. This could be attributed to the increase of reaction temperature that normally causes the decrease in the amount of stabilizer agent available on the droplet surface due to its increasing solubility in water [15], leading to a greater coalescence of polymer droplets and, therefore, the increase of polystyrene content and the decrease of the proportion between PRS® paraffin wax and the polymer shell in the GPC and DSC analyses.
On the other hand, it can be observed (Fig. 4) that as the reaction temperature increases the molecular weight also grows and the molecular weight distribution of polystyrene becomes narrower (polydispersity index). Molecular weights of samples varied between Mn = 11,791 and Mw = 24,256 g/mol at 98 °C and Mn = 23,629 and Mw = 43,670 g/mol at 113 °C. This fact is related to the use of BPO as initiator and styrene as monomer. BPO has a chain-transfer constant relatively large (0.048–0.10) at 60 °C for polymerization of the styrene because of the high reactivity of their propagating radicals [14].
This behavior has been observed by other authors on polystyrene synthesis using BPO. Higher polymerization temperatures caused the system to run out of initiator after a very high initial polymerization rate and lower terminal conversions were reached. For these inefficient polymerizations, higher molecular weights were obtained at terminal conversion [16].
Most practical applications of microparticles require higher molecular weights to obtain higher strengths. For this reason, the molecular weight of the polymeric shell is of prime importance in the synthesis of microcapsules for its further application [17]. A higher capsule hardness will allow a thick shell with enough physical resistance. The other target is to encapsulate the maximum amount of PCM in the same volume to have large energy microcapsule storage. Although an extensive study of hardness of microcapsules by using atomic force microscopy is required, we should consider that 108 °C shows a good compromise between shell resistance and PCM content and is appropriate to study the other synthesis variables.
Influence of stirring rate
Experiments varying the stirring rate were carried out in the experimental setup shown in Fig. 1. Stirring rate strongly affects the suspension polymerization since it modifies the oil phase dispersion. Polymerization could yield powder or granules depending on the stirring conditions used.
The results shown in Fig. 5 indicate that the particle size will be smaller if the energy delivered by the stirrer is greater. Obviously, the case of capsule formation follows the same rule of suspension polymerization process. According to Zhang et al. [18], the diameter of the microcapsules decreases with the increase of the stirring rate. It is well known that stirring rate affects both the formation of drops and the aggregation through collisions of neighboring globules. Obviously, under vigorous stirring, the aggregations of globules are minimized, obtaining then the lowest particle size (Fig. 5).
The particle size is one of the most important properties that have to be taken into account for the application of PCM microcapsules for concrete use. In the case of textile application, microcapsules with low particle sizes are required. In this sense, it would be important to find a relationship between the stirring rate and the particle size. The experimental values obtained have been fitted to the following power law equation:
where dpn0.5 is the number average diameter of microparticles in micrometers and N is the stirring rate in revolutions per minute; the coefficient of determination (R 2) is 0.998.
The value of the exponent was higher than that previously reported in the literature about suspension polymerization process where the common value used to be about −1.5 [19, 20]. This fact could be related to the viscosity of the liquid medium in the suspension process which is only due to styrene at 108 °C (0.4 cSt). Nevertheless, in the microencapsulation process, the viscosity is due to styrene and paraffin wax at 108 °C (1.102 cSt). For that reason, the horsepower requirements of the agitator motor should increase as the viscosity increases. Nevertheless, the mathematical relation of particle diameter with the stirring speed follows a similar rule with that in a conventional suspension polymerization system and is in good agreement with the type of equation used in those cases [19].
A stirring rate of 900 rpm was selected for the following experiments because the average particle diameter of microcapsules (around 30 μm) can be adequate for the textile application and the operation conditions are not very severe.
Influence of the mass ratio of PRS® paraffin wax to styrene
Thermoregulatory properties of the microcapsules depend on the relative amount of PCM encapsulated with respect to the shell weight and use to be expressed as the energy storage capacity per mass unit [21]. In order to explore the possibility of encapsulating a greater amount of paraffin per mass of polymer, a series of experiments using different core to coating ratios in the range from 0.35 to 2.00 were carried out.
The experiment carried out with a mass ratio of 2.00 (not shown) was not successful for the microencapsulation process. This fact could be a consequence of a shortage of polymer that could not completely cover the amount of paraffin added.
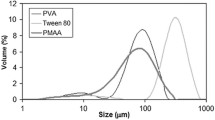
PSDs in volume of the materials obtained after the polymerization process using different PRS® paraffin to styrene mass ratios are shown in Fig. 6. The PSD of microparticles obtained exhibits a unimodal distribution, with not very narrow distribution of particle sizes and a low percentage of large particles (>1 mm) for all PRS® paraffin/styrene mass ratios studied. These results indicate that the average diameter in volume of the microcapsules increases with the increase of paraffin core/polymer shell mass ratio. This fact could be because the viscosity of the mixture forming droplets is smaller as the monomer content at the beginning of the reaction is greater. Therefore, the diameter of the microparticles enlarges when the viscosity of the mixture increases.
Due to greater heterogeneity of particle size, microcapsules obtained with mass ratios of 0.50 and 1.00 were sieved in order to analyze particles with different sizes by DSC. In Fig. 7, the effect of core/shell mass ratio is shown, together with the latent heat versus particle size of microparticles obtained. The results showed that, with the increase of particle size, the proportion of the paraffin contained in the particles decreases. Furthermore, it can be seen that, when the paraffin/styrene mass ratio of 0.50 is used, the effect on proportion of encapsulated paraffin is even greater. The melting heats per mass unit of those particles were 153.5, 135.6, 80.8, and 41.7 J/g corresponding to microcapsules of 250, 500, 1,000, and 2,000 μm, respectively.
The DSC thermograms of the smallest microparticles obtained at different PRS® paraffin to styrene mass ratios used in the experimental study are shown in Fig. 8. The class of the microcapsules analyzed was those with a diameter lower than 250 μm because the size of the microcapsules should be lower for its further application in the textile industry. As the core to coating ratio increases, the amount of paraffin wax encapsulated increases passing through a maximum and then decreases again.
The measured paraffin wax content in the microcapsules according to DSC measurements is totally different from the theoretical contents included in the recipe. It means that not all the paraffin is successfully encapsulated; there is remaining free paraffin in the bulk at the end of the reaction process. However, the differences between the measured and theoretical contents from the recipe are not only caused by the efficiency of the encapsulation but also by the heterogeneous size distribution and the morphology of microcapsules obtained. These facts are logically related to a higher encapsulation efficiency of the paraffin wax inside the microparticles.
On one hand, these results could be related to the initial period of polymerization process where the monomer is subjected either to turbulent pressure fluctuations or viscous shear forces [22]. Before reaching the identity point, during the period where the coalescence and breakage of droplets happens, the transfer of paraffin wax and monomer between the droplets is not uniform. The initially formed polymer tends to attract more monomer for the formation of larger particles and, as a consequence, a major proportion of paraffin wax is encapsulated in the smallest particles. That is confirmed by the observations: the smaller the size of the particles, the higher the melting heat of the microcapsules.
On the other hand, the experimental results can be explained by considering the agglomeration between smaller microparticles for the formation of the larger microcapsules. Figure 9 shows the internal structure of a large microparticle obtained using a mass ratio of 0.35 by cross-sectioning the microcapsule, and a higher magnification of the inner structure of the microtomed microcapsule (included in Fig. 9) confirms that smaller microcapsules with paraffin wax inside, polystyrene particles, and paraffin matrix are agglomerated inside of the larger one. This fact could confirm the small quantity of paraffin encapsulated that presented the larger particles in DSC analyses (Fig. 7).
Moreover, taking into account that the styrene–paraffin wax system has no large driving forces to promote phase separation because the values of polarity and interfacial tensions of polystyrene are quite similar to paraffin wax, then the core/shell morphology could not be thermodynamically favored during polymerization process. According to Sundberg et al. [23], if γ wo > (γ wp + γ op), a core/shell particle would be obtained over any of the other possible morphologies (as salami-like structure), in which γ wo is the interfacial tension of the water and the oil interphase, γ wp is the interfacial tension of the water/polymer interface, and γ op is the interfacial tension of the oil/polymer interface.
As it has been said, the PCM content of the microcapsule has to be as high as possible and strong enough to provide proper capsule resistance [7]. So, the highest amount of paraffin wax encapsulated was 75.6 wt.%, which is much higher than some of the best values obtained for microcapsules of PCMs with a similar average particle size but manufactured by other methods.
To sum up, the PRS® paraffin/styrene mass ratio of 0.50 would be the best choice to encapsulate a high proportion of paraffin. Above that, with a mass ratio of 1.00, the encapsulation process is not possible. Furthermore, the microcapsules obtained present suitable particle sizes and an excellent thermal capacity to be applied in fabrics.
Conclusions
Microcapsules containing PRS® paraffin wax and a polystyrene shell were synthesized using different synthesis conditions by means of suspension-like polymerization technique. The factors influencing thermal storage capacity, particle size distribution, and morphology were investigated. The reaction temperature has no effect on the particle size distributions; however, it has significant effect on the molecular weight. As stirring rate increased, the diameter of microcapsules decreased due to the monomer droplet break-up and aggregation, which were then equilibrated rapidly and suspensions thus became stable. Temperature synthesis between 98 °C and 113 °C and higher stirring rate (900 rpm) were the most adequate parameters for the microencapsulation of PRS® paraffin with polystyrene as shell.
In order to improve the thermoregulating efficiency of the microparticles, the effect of core/shell mass ratio was studied. The results indicate that the average diameter in volume of the microcapsules increases with the increase of paraffin core/polymer shell mass ratio; however, the melting heat decreased. Smallest particles showed the highest amount of PCM inside. Paraffin wax was difficultly encapsulated when the mass ratio of PRS® paraffin wax to styrene was higher than 1.00. The highest amount of paraffin wax encapsulated was 75.6 wt.%, which was higher than that of the microcapsules with a similar average particle size manufactured by other methods.
References
Benita S (1996) Microencapsulation: methods and industrial applications. Dekker, New York
Zalba B, Marín JM, Cabeza LF, Mehling H (2002) Appl Therm Eng 23:251–283
Farid MM, Khudhair AM, Razack SAK, Al-Hallaj S (2004) Energy Convers Manag 48:1597–1615
Nelson G (2007) Int J Pharm 242(1–2):55–62
Han N, Zhang X, Wang X (2007) J Appl Polym Sci 103:2776–2781
Ghosh SK (2006) Functional coating by polymer microencapsulation. Wiley-VCH, Belgien, p 224
Shin Y, Yoo D, Son K (2005) J Appl Polym Sci 96:2005–2010
Su J, Wang L, Ren L (2006) J Appl Polym Sci 101:1522–1528
Sanchez L, Sanchez P, de Lucas A, Carmona M, Rodriguez JF (2007) Colloid Polym Sci 285:1377–1385
Xing L, Hongyan L, Shujun W, Lu Z, Hua C (2006) Sol Energy 80:1561–1567
Zheng LH, Song GS (2003) J Wuhan Polytechnic University 22:54–57
Zou GL, Lan XZ, Tan ZC, Sun LX, Zhang T (2004) Acta Phys-Chim Sin 20:90–93
Dobiáš B, Rybinski W (1999) Solid–liquid dispersions. CRC, p 41
Odian G (2004) Principles of polymerization. Wiley, New York, pp 234–235
Shen S, Sudol ED, El-Aasser MS (1993) J Polym Sci Part A: Polym Chem 32:1087–1100
Villalobos MA, Hamielec AE, Wood PE (1991) J Appl Polym Sci 42:629–641
Su J, Wang L, Ren L, Huang Z (2006) J Appl Polym Sci 103:1295–1302
Zhang XX, Fan YF, Tao XM, Yick KL (2004) Mater Chem Phys 88:300–307
Hopff VH, Lüssi H, Gerspacher P (1964) Makromol Chem 78:37
Cordoví C, de Lucas A, Durán A, Rodríguez JF (2000) J Appl Polym Sci 76:814–823
Sarier N, Onder E (2006) Thermochim Acta 452:65–76
Vivaldo-Lima E, Wood PE, Hamielec AE (1997) Ind Eng Res 36:939–965
Sundberg D, Casassa AP, Pantazopoulos J, Muscato MR (1990) J Appl Polym Sci 41:1425–1442
Acknowledgments
The financial support from ASINTEC S.A. and a grant from Consejería de Ciencia y Tecnología (JCCM), respectively, are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sánchez, L., Sánchez, P., Carmona, M. et al. Influence of operation conditions on the microencapsulation of PCMs by means of suspension-like polymerization. Colloid Polym Sci 286, 1019–1027 (2008). https://doi.org/10.1007/s00396-008-1864-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-008-1864-4