Abstract
A novel cheap macromolecular intumescent flame retardants (MIFR) was synthesized, and its structure was a macromolecule containing phosphorus characterized by IR. Rigid polyurethane foam (PUF) filled with MIFR as fire retardant additive was prepared. The effects of MIFR on properties such as density, compressive strength, flame-retardant behavior, thermal stability, and morphology of char were studied. The compressive strength of the MIFR-filled PUF increased initially and then decreased with further increase of MIFR content while its density straightly increased. Its flammability and burning behavior were characterized by UL 94 and limiting oxygen index (LOI). Twenty five percent of MIFR was doped into PUF to get 24.5 of LOI and UL 94 V-0. Activation energy for the decomposition of samples was obtained using Kissinger equation. The resultant data show that for PUF containing MIFR, compared with PUF, the mass loss, thermal stability, and the decomposition activation energy decreased, the char yield increased, which shows that MIFR can catalyze decomposition and carbonization of PUF to form an effective charring layer to protect the underlying substrate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, halogen-free intumescent flame retardant (IFR) is attracting more and more attention from both academic and industrial communities for their multifold advantages including low toxic, low smoke, low corrosion, no corrosive gas, and so on [1, 2]. Three ingredients are necessary for IFR: acid source, carbon source, and gas source. Phosphorus-containing compounds are often used as an acid source, while nitrogen-containing compounds are used as a blowing agent. The greatest benefit to be obtained in this way is a dramatic decrease in the heat generated due to the exothermic combustion of polymers. Other advantages include the conservation of the structural integrity of polymer as a result of the residue of solid carbon and a decrease of formation of flammable gaseous products [3]. In the former work of our group, a novel macromolecular IFR (MIFR) which contains an acid source, a gas source, and a char source simultaneously had been synthesized by pentaerythritol, phosphoric acid, melamine (M), urea (U), and formaldehyde (F) [4, 5] and applied to epoxy resin to get good flame-retardant (FR) efficiency [6]. However, pentaerythritol, as an important raw material in MIFR, is expensive. It is well known that starch could be the carbon source of IFR instead of pentaerythritol. It was recently reported that introduction of starch had an obvious effect on the structure of the intumescent and charry layers [7].
So in this work, an improved MIFR was firstly synthesized using starch as char source instead of pentaerythritol, which was applied to rigid polyurethane foams (PUF). The effects of MIFR on properties such as density, compressive strength, FR behavior, thermal stability, and morphology of char were investigated.
Experimental
Materials
Polymeric methane diphenyl diisocyanate (PMDI, PM-200, NCO = 30.2–32.0 %) was obtained from Yantai Wanhua polyurethane Co. Ltd.; poly(ether polyol) (NT-4110,OH content = 410 mg of KOH g−1) was obtained from LAN-STAR East Chemical Co. Ltd.; Silicone glycol copolymer (SGC), a surfactant, was purchased from Shanghai Chemical Reagent (Shanghai, China). Dibutyltin dilaurate (DBDT), a tin catalyst with a density of 1.052 g cm−3 and an Sn content of 18 mass%, and triethylamine, amine catalyst, were purchased from Sichuan Chemical Reagent (Sichuan, China). Distilled water as blowing agent was prepared by ion-exchange unit. Starch, 85 % of phosphoric acid, M, 37 % of formalin as F, and U were received from Tianjin Fuchen Chemical Reagent Factory.
Synthesis of MIFR
MIFR was synthesized as Ref. [4], firstly, amino resins prepolymer was prepared, then the amino resins prepolymer was polycondensated under acidic starch phosphate, the reaction is complicated, one of the possible reaction is to see Scheme 1. Thirty seven percent of formalin as F 1 mol was brought to pH 8–8.5 with NaOH and heated. Then, M 0.2 mol and U 0.5 mol were added to the above solution, stirred until dissolved, and heated under reflux for 50 min. Heating was stopped, and the solution was allowed to cool to get MUF prepolymer A. 10 mL of 85% phosphoric acid and 25 g of starch were mixed, heated to 373 K for 30 min, added 15 mL phosphoric acid after cooled to 343–353 K, reacted for 30 min to get an acidic starch phosphate B. B was added slowly into A under stirring to obtain MIFR. The synthesis route is shown in Scheme 1.
Preparation of the foam
Polyols 4110, distilled water, catalysts (DBDT and triethylamine), surfactant (SGC), and flame retardant (MIFR) were well mixed in a 1 L beaker. Next, PMDI was added into the beaker with vigorous stirring for 10 s. The mixture was immediately poured into an open mold (300 × 250 × 150 mm3) to produce free-rise foam. Foam blocks so obtained were kept in an oven at 343 K for 24 h to complete the polymerization reaction. Samples were cut into the desired shape and size by rubbing with fine emery paper, and these test species were used for the evaluation of different properties. The formulations of PUF are shown in Table 1.
Characterization
Mechanical properties
The compressive properties were tested with a CMT4204 universal testing machine (Meitesi, USA) according to GB/T 8813-2008. The apparent density of the PUF samples was measured as ASTM D 1622-03. At least three samples were tested to obtain average values.
Vertical burning tests
The vertical burning test was conducted by a CZF-II horizontal and vertical burning tester (Jiang Ning Analysis Instrument Company, China). The specimens which used were 130 × 12.7 × 3 mm3 according to UL94 test ASTM D3801 standard. The procedures of the standard vertical burning test as well as the definitions and units of Vertical burning rate were referred to what were expatiated in the literature [8].
Limiting oxygen index test
The limiting oxygen index (LOI) test was performed with a JF-3 oxygen index test instrument (Jiangning, China) in terms of the standard LOI test, ASTM D 2863-97. The specimen size for the LOI measurement was 127 × 10 × 10 mm3.
Scanning electron microscope (SEM) analysis
The char residues after LOI test were studied with a scanning electron microscope (SEM) (KYKYEM-3200, China) SEM. The samples were gold-coated before scanning to provide an electrically conductive surface. An accelerating voltage of 22 kV was used, while we recorded the scanning electron micrograms.
Thermogravimetry analysis
Thermogravimetry (TG) was carried out on a HCT-2 thermal analyzer (Beijing Hengjiu Scientific Instrument Factory) under a dynamic nitrogen (dried) atmosphere at a heating rate of 10 K min−1, and 4 mg samples were heated from room temperature to 1023 K.
IR spectra
The IR spectra were measured on an IS5 (Nicolet) spectrophotometer in the range of 4,000–600 cm−1 at a resolution of 0.5 cm−1. Samples were ground and mixed with KBr to form pellets.
Characterization of MIFR
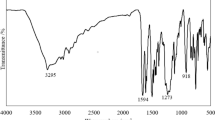
IR (KBr) to see Fig. 1 (cm−1) is 3094 (–OH, NH), 2949, 2877 (w, CH2), 1254 (P=O), 1506, 814 (C=N), 1127, 982, 618 (P–O–C).
Results and discussion
Mechanical properties
The mechanical properties of PUF are important parameters that determine its applications, such as in load bearing and as packaging materials. Foam density is a very important parameter that affects the mechanical properties of PUFs [9]. In general, the foam density is dependent on the degree of foaming, which in turn depends in part on the type and amount of blowing agent. In this study, the amount of chemical blowing agent was kept constant. Figure 2 shows the density of PUFs filled with MIFR at different loadings. It indicates that the density increased with the increasing MIFR loading. This was due to a decrease in the cell size and to the higher density of MIFR than that of neat PUF.
To study the effect of MIFR loading on the compressive properties of PUFs, the compressive strength of the foams containing different MIFR loadings was determined and shown in Fig. 3. Figure 3 shows the effects on the compressive strength of the PUFs filled with the increasing loading of MIFR. Figure 3 indicates that the compressive strength initially increased and then decreased with further increases in the MIFR loading. The initial increase in properties was due to an increase in the cell wall thickness and also an increase in the density. It is known that the degree of foaming of PUFs depends on the viscosity and surface tension of the particular formulation [10]. Addition of MIFR resulted in an increase in the viscosity, and this led to a decrease in the blowing or expansion of the PUFs and an increase in the density. The mechanical properties of PUF filled with higher loadings of MIFR (above 20 %) were decreased. This decrease in the mechanical properties was due to its poor wetting or adhesion with the polymer matrix, and also, with inclusion of higher amounts, and leads to agglomeration because the filler–filler interaction becomes more pronounced, which agrees with the reports [11].
Flame retardancy of rigid polyurethane foams
LOI is a fundamental tool in basic research on polymer combustion and mechanism of fire retardancy. LOI also provides an evaluation of intrinsic flammability of the polymeric materials [12]. Thus, flammability of PUF samples containing different MIFR is evaluated by determining their LOIs, and the values are presented in Table 2. LOI of PUF is 18.1 which increases from 20.5 to 24.5 % as the MIFR content is increased from 10 to 25 %. A LOI of PUF containing different MIFR is increased due to the presence of phosphorus and nitrogen in MIFR. Phosphorus and nitrogen have synergistic effect between them which enhances the LOI of PUF. The trend of these data shows that LOI increases with the increasing concent of MIFR. The rigid PUF obtained qualified for the UL 94 V-0 rating at low phosphorus contents of 3.60 % phosphorus with 25 % mass of MIFR.
Degradation of rigid polyurethane foams
The simultaneous DTG and TG curves of PUF-1 and PUF-5 were carried out in dynamic nitrogen from ambient temperature to 1023 K and are shown in Fig. 4. The initial decomposition temperature (IDT) determined by 5 % of mass loss, integral procedure decomposition temperature (IPDT) determined by 50 % of mass loss, and char yield at 973 K were measured, listed in Table 3.
When the FR elements are incorporated into polymeric materials, the mass loss pattern of the polymers is altered. Phosphorus groups decompose at relatively low temperature to form a heat-resistant char, to retard the mass loss rate of the polymers at high temperatures [13]. Nitrogen-containing compounds produce incombustible gases when they degrade. The heat gases can swell the just formed char. The swollen char can isolate the transfer of mass and heat between the materials and the flame in a fire. That protects the unburned materials. The actions play some critical roles in flame retarding polymeric materials through condensed-phase mechanisms as well as gas-phase mechanism.
From Fig. 4, it can be seen that there is a main and quick decomposition stage, and the mass loss behavior of PUF containing MIFR is found to follow the patterns discussed above. From Table 3, For PUF containing MIFR (PUF-5), compared with PUF-1, char yields (39.1 %) are increased. The decrease significantly of mass loss rates lowers the amount and rate of release of combustible products from the rigid PUF’ decomposition, consequently depressing the resins’ flammability. The increase of char yields agrees with mechanism of FR [14]. Introduction of FRs leads to more char formed at the expense of flammable volatile products of thermal degradation, thus suppressing combustion and increasing the LOI.
Thermal stability of rigid polyurethane foams
The thermal stability of the rigid PUF is assessed with two parameters: IDT and IPDT. IDT indicates the apparent thermal stability of the rigid PUF, i.e., the failure temperatures of the resins in processing and molding. On the other hand, IPDT exhibits the resins’ inherent thermal stability, i.e., the decomposition characteristics of the resins’ volatile composition. From Table 3, phosphorus-containing rigid PUF (PUF-5) shows relatively lower IDT than do the phosphorus-free resin (PUF-1), since phosphorus groups decompose at low temperatures. On the other hand, the existence of MIFR (PUF-5) exhibits higher IPDT than the PUF-1, retarding the mass loss rate of the polymers at high temperatures. The high IPDT (729 K) implies the rigid PUF’ potential application in highly anti-thermal coatings and thermal insulating materials.
The decomposition activity energies
The decomposition activity energies of PUF-1 and PUF-5 were studied by the equation of Kissinger [15]. The equation is as follows:
where Φ is the rate of temperature increase in K min−1 (Φ = 2, 5, 10, 20), T m is the maximum temperature at the peak position in K, E a is the decomposition activity energy, and R is the gas constant (8.314 J mol−1 K−1). From the slope of the plot of ln(Φ/T 2m ) versus 1/T m, E a can be calculated, i.e., E = R × slope. Table 3 presents the activation energies (E a) for PUF-1 and PUF-5.
E a for the decomposition of PUF is 205 kJ mol−1, while it becomes 162 kJ mol−1 when MIFR is doped into, decreased by 43 kJ mol−1, which shows that MIFR can catalyze decomposition and carbonization of PUF. It is supported by the lower IDT (420 K).
Figures 5 and 6 present the SEM photographs of the surface of char of the PUF composites (PUF-1, PUF-5). From Fig. 5, it can be observed that the char is very slight, loose, and soft, which could not protect the underlying material from fire. Contrarily, Fig. 6 shows that the char is compact, tough, and hard though there are some holes on the surface. It is possible that the MIFR could promote the formation of effective charring layer. The structure of the intumescent charring layer may increase the efficiency of the flame retardancy, act as heat insulation, and protect inner matrix materials.
Conclusions
We succeed in synthesizing a novel cheap macromolecular MIFR with a structure of a starch diphosphonate. The compressive strength of the MIFR-filled PUF increased initially and then decreased with further increase in MIFR content, while its density straightly increased. Twenty five percent of the MIFR is doped into PUF to get 24.5 of LOI and UL 94 V-0. For PUF containing MIFR, compared with PUF, incorporating MIFR into rigid PUF alters degradation characteristics, which decreases mass loss, increases the char yield. However, for PUF containing MIFR, the IDT and activation energy are decreased, which shows that the stability was decreased. E a for the decomposition of PUF is 205 kJ mol−1, while it becomes 162 kJ mol−1 for PUF containing MIFR, decreased by 42 kJ mol−1, which shows that IFR can catalyze decomposition and carbonization of PUF to form an effective charring layer. In the thermal degradation of PUF containing MIFR, phosphorus groups decompose at relatively low temperature, then catalyzing decomposition and carbonization of PUF to form a heat-resistant char, retarding the mass loss rate of the PUF at high temperatures. Nitrogen serving as blowing agents and char-reinforcing components leads to the production of intumescent chars, which protects the underlying combustible substrate to get good flame retardancy.
References
Nie S, Peng C, Yuan S, Zhang M. Thermal and flame retardant properties of novel intumescent flame retardant polypropylene composites. J Therm Anal Calorim. 2013;113(2):865–71.
Sun L, Qu Y, Li S. Co-microencapsulate of ammonium polyphosphate and pentaerythritol in intumescent flame-retardant coatings. J Therm Anal Calorim. 2013;111(2):1099–106.
Yuan D, Yin H, Caia X. Effect of a novel flame retardant containing silicon and nitrogen on the thermal stability and flame retardancy of polycarbonate. J Therm Anal Calorim. 2013;111(2):1531–7.
Gao M, Wu W, Yan Y. Thermal degradation and flame retardancy of epoxy resins containing intumescent flame retardant. J Therm Anal Calorim. 2009;95:605–8.
Gao M, Yang SS. A novel intumescent flame-retardant epoxy resins system. J Appl Polym Sci. 2010;115:2346–51.
Gao M, Wu W, Xu Z. Thermal degradation behaviors and flame retardancy of epoxy resins with novel silicon-containing flame retardant. J Appl Polym Sci. 2013;27:1842–7.
Wang X, Feng N, Chang SQ. Intumescent flame retardant TPO composites: flame retardant properties and morphology of the charred layer. J Appl Polym Sci. 2012;124(3):2071–9.
Bian XC, Tang JH, Li ZM, Lu ZY, Lu A. Dependence of flame-retardant properties on density of expandable graphite filled rigid polyurethane foam. J Appl Polym Sci. 2007;104:3347–55.
Thirumal M, Khastgir D, Singha NK, Manjunath BS, Naik YP. Effect of foam density on the properties of water blown rigid polyurethane foam. J Appl Polym Sci. 2008;108:1810–7.
Modesti M, Lorenzetti A. Flame retardancy of polyisocyanurate-polyurethane foams: use of different charring agents. Polym Degrad Stab. 2002;78:341–7.
Harpal S, Jain AK, Sharma TP. Effect of phosphorus-nitrogen additives on fire retardancy of rigid polyurethane foams. J Appl Polym Sci. 2008;109:2718–28.
Camino G, Costa L, Casorati E, Bertelli G, Locatelli R. The oxygen index method in fire retardance studies of polymeric materials. J Appl Polym Sci. 2003;35(7):1863–76.
Youssef B, Mortaigne B, Soulard M, Saiter JM. Fireproofing of polyurethane by organophosthonates. J Therm Anal Calorim. 2007;90(2):489–94.
Kandola BK, Horrocks AR, Price D, Coleman GV. Flame-retardant treatments of cellulose and their influence on the mechanism of cellulose pyrolysis. J Macromol Sci Rev Macromol Chem Phys. 1996;C36(4):721.
Kissinger HE. Reaction kinetics in differential thermal analysis. J Res Natl Bur Stand. 1956;57:217–21.
Acknowledgements
The work was supported by Hebei Province Natural Science of China (E2011508001) and Fundamental research funds for the Central Universities (3142013102).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, M., Wu, W., Liu, S. et al. Thermal degradation and flame retardancy of rigid polyurethane foams containing a novel intumescent flame retardant. J Therm Anal Calorim 117, 1419–1425 (2014). https://doi.org/10.1007/s10973-014-3856-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-3856-6