Abstract
A novel flame retardant (PSiN), containing silicon and nitrogen, was synthesized using N-(β-aminoethyl)-γ-aminopropylmethyldimethoxysilane and diphenylsilanediol through solution polycondensation and it was added to polycarbonate (PC). The structure and thermal properties of PSiN were characterized by fourier transform infrared spectroscopy and thermogravimetric analysis (TG) tests. The effect of PSiN on the flame retardancy and thermal behaviors of PC was investigated by limited oxygen index (LOI), vertical burning test (UL-94), and TG tests. The results showed that the flame retardancy and the thermal stability of PC are improved with the addition of PSiN. When 1 mass% PSiN and 0.5 mass% diphenylsulfone sulfonate (KSS) are incorporated, the LOI value of PC is found to be 46, and class V-0 of UL-94 test is passed. The char structure observed by scanning electron microscopy indicated that the surface of the char for PC/KSS/PSiN system holds a firmer and denser char structure when compared with neat PC and PC/KSS system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycarbonate (PC), one of the fastest growing engineering polymers, is widely used in construction, medical equipment, transport, and other fields as to its excellent mechanical properties, outstanding electrical properties, high glass transition temperature, and so on [1, 2]. PC is a naturally high charring polymer, showing a V-2 rating in the vertical burning test (UL-94). However, strict flame retardant performance is often required for electronic and electric applications, so the flame retardancy of PC still needs to be improved [2–4].
Traditionally, halogenated flame retardant was widely incorporated into PC to improve its flame retardant performance. However, it releases toxic gases and corrosive smoke during combustion [5–7], so it has given rise to some environmental concerns. Accordingly, to develop non-halogenated flame-retardant system becomes an attractive and emergent subject [8, 9].
In 1970s, it has been reported that sulfosalt was absolutely beneficial for the flame retardancy of PC. Innes et al. [10] studied the effect of flame retardancy of potassium butylperfluorosulphonate (KBPFS) for PC. It was reported that when 0.5 % potassium diphenylsulfone sulfonate (KSS) was incorporated into PC, the limited oxygen index (LOI) value of PC could achieve at 25–35 % [11, 12]. However, the content of KSS in PC was generally less than 1 % because the addition of KSS could reduce the hydrolytic stability of PC.
Recently, silicon-containing compounds used as flame retardant have received more and more attentions owing to the environmentally-friendly consideration [13–15]. For example, polysilsesquioxanes with (RSiO3/2)n formula, where R is an organic substituent, have been used as flame retardants due to its excellent flame retardancy, high impact resistance, superior moldability, recyclability, and the potential to replace halogen-containing flame retardants [16–18]. He et al. [19, 20] investigated the synergistic effects of polyhedraoligomeric silsesquioxane (POSS) and oligomeric bisphenyl A bis(diphenyl phosphate) (BDP) on thermal and flame retardancy of PC. Periadurai et al. [21] reported thermal decomposition and flame-retardant behaviors of SiO2-phenolic nanocomposite.
Owing to the presence of phenyl, diphenylsilanediol is inferred to be compatible with PC. N-(β-aminoethyl)-γ-aminopropylmethyldimethoxysilane (KH-602) is a kind of organic silicon with nitrogen, which may have excellent flame retardancy. As is known, the flame-retardant efficiency is in connection with compatibility and reactivity; hence, we synthesized a novel flame retardant (PSiN) using diphenydidroxysilane and KH-602 through solution polycondensation and it was added to PC system. The burning behaviors, thermal stabilization, and char formation were investigated.
Experimental
Materials
PC (S23001R) was purchased from Mitsubishi Engineering Plastics Corporation (Japan). KSS was supplied by Sloss Industries Corporation (USA). Antioxidizer (1010) was provided by Tianjing Chemical Factory (Tianjing, China). KH-602 and diphenylsilanediol were acquired from Nanjing Union Silicon Chemical Co. Ltd (Nanjing, China) and Bluestar Chemical Research Institute (Chengdu, China), respectively.
Synthesis of PSiN
A 150 mL three-necked round bottom flask equipped with a stirrer and a reflux condenser was charged with diphenylsilanediol (25.96 g, 0.12 mol), toluene (36 mL), and HCl (0.0021 mol, 10.5 mL of 0.2 mol L−1). Then, the mixture was stirred until diphenylsilanediol dissolved completely. After that, 18.56 g (0.09 mol) KH-602 was added slowly by drops to the flask within about 0.5 h and the temperature was kept at 55 °C. Then, the flask was heated up to 85 °C and kept refluxed until there is no increase of viscosity for the reaction system. After being cooled to room temperature, the raw product was neutralized by sodium hydroxide (NaOH) and washed by water to remove the residual KH-602 and HCl, then mixture was washed by ethanol to eliminate toluene and remaining water. The product was dried in a vacuum at 110 °C. (Product yield: 55.6 %). The synthesis route was illustrated in Scheme 1.
Preparation of flame-retardant PC samples
PC, dried at 120 °C for 6 h, antioxidizer (1010), KSS and PSiN were extruded into pellets in a double-screw extruder (TSSJ-25, Bluestar Chemical Research Institute, Chengdu, China) at a temperature range of 235–250 °C based on a calculated amount of ratio. Then, the extruded composites were dried at 120 °C for 7 h. The well dried pellets were added into the injection-molding machine (NISSAN PS40E5ASE) and molded into standard testing bars for further test.
Measurements
IR spectroscopy was applied with a Nicolet IS10 fourier transform infrared (FTIR) spectrometer using KBr pellets. LOI data of all samples were obtained at room temperature on an oxygen index instrument (XYC-75) produced by Chende Jinjian Analysis Instrument Factory, according to ASTM D2863-77 standard. The dimensions of all samples are 130 × 6.5 × 3 mm3. Vertical burning rates of all samples were measured on a CZF-2 instrument produced by Jiangning Analysis Instrument Factory, with sample dimensions of 125 × 12.5 × 3.2 mm3, according to UL-94 test ASTM D635-77. The surface morphology of the char obtained after the LOI test was observed using a Inspect-F scanning electron microscope (SEM).
All thermogravimetry analysis (TG) tests were performed on a Mettler Toledo TG/DSC1 instrument thermal analyzer. About 10 mg of a tested sample was heated from 50 to 800 °C under neat nitrogen at rates of 5, 10, 15, and 20 °C min−1, respectively. The temperature reproducibility of the TG is ±3 °C and error range of the mass is ±3 %. The thermal degradation activation energy was determined directly from mass loss versus temperature data obtained at several heating rates by the Ozawa method [22–24]. According to this method, the equation of thermal decomposition can be expressed as follows:
where r is the heating rate, T is the absolute temperature at the different heating rate under the same mass loss, E is the activation energy of the decomposition reaction, and R is the gas constant. The above equation shows that log r is linearly proportional to 1/T. The activation energy can then be determined by a calculation of the slope from the log r−1/T plots.
Results and discussions
Characterization
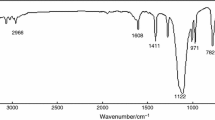
Figure 1 presents the FTIR spectra of the synthesized PSiN. The absorption bands at 1,674 and 3,070 cm−1 correspond to vibrations of the benzene rings. The peaks at 1,132 and 1,047 cm−1 are associated with the vibrations of Si–O–Si. The absorptions at 2,958, 1,261, and 804 cm−1 are assigned to Si–CH3. Furthermore, the alcoholic peak of diphenylsilanediol is almost disappeared. The information above confirms that the target product is synthesized successfully.
Flame retardancy
A series of flame retardants differing in PSiN to KSS ratio were manufactured and they were compounded with PC. Table 1 shows the LOI values and UL-94 test for PC compositions. From Table 1, LOI values of the PC/KSS system without PSiN and the PC/PSiN system without KSS are 33 and 31, respectively; both of them just get V-1 class in UL-94 test. This demonstrated that KSS and PSiN shows low efficiency in flame retardancy of PC alone. However, when the mixture of KSS and PSiN is incorporated into PC, the LOI values are dramatically increased. For example, when 3 mass% PSiN and 0.5 mass% KSS are added, the LOI value of PC is found to be 47, and class V-0 of UL-94 test is passed. Moreover, the flame retardancy of PC through PSiN and KSS is not increasing linearly with their content, but favouring small amounts and there is a pronounced synergy of KSS and PSiN. In addition, PSiN with excellent flowability can spread to outside from inside during combustion and accumulate on the surface to act as a thermal insulation layer, creating a less flammable material [25]. Both reasons together resulted in very good LOI and UL-94 performance with very small amount of flame retardant.
Thermal stability
Figure 2 shows the TG and DTG curves of PSiN. As can be seen, there is a slightly degradation peak around 110 °C, which possibly attributes to the water evolved in the reaction or absorbed in the medium. PSiN is thermally stable below 310 °C, then it undergoes a rapid mass loss in the range 320–500 °C. The max degradation temperature (T max) of PSiN is 410 °C, which is higher than those of conventional phosphorus-containing flame retardants (BDP, RDP, and TPP) and closes to the onset degradation temperature (T 5 mass%) of PC, indicating that PSiN may better exert its flame-retardant action before the fast degradation of PC. When the temperature reaches 800 °C, PSiN almost decomposes completely and only 9.51 % char residue exists.
Figure 3 shows the TG curves of experimental and calculated KSS/PSiN (1/1) mixture. According to Fig. 3, one can observe the remarkable difference between the experimental and calculated TG curves of KSS/PSiN. The calculated curve of KSS/PSiN occurs one thermal degradation process at 320–500 °C and the T max is 417 °C. However, the experimental curve of KSS/PSiN exhibites two thermal degradation steps at 320–500 °C. The decomposition temperature in the first step is at 320–375 °C; the second decomposition step is ranging from 437 to 500 °C, and the main mass loss occurs in the second stage. The char formation efficiency of KSS/PSiN experimental above 420 °C is much higher than that of KSS/PSiN calculated. The char residue of KSS/PSiN experimental is 47.5 % at 700 °C, while it is only 32.1 % based on calculation. It proposes that there is an interaction between KSS and PSiN when the temperature is above 350 °C and forms a relatively stable chemical structure and promotes char formation at high temperature.
The TG curves for PC/KSS and PC/KSS/1 mass% PSiN systems observed at different heating rate are given in Figs. 4 and 5, respectively. Tables 2 and 3 shows the corresponding characteristic mass loss data. The curves of both PC/KSS and PC/KSS/1 mass% PSiN systems show a one-step degradation process. The onset degradation temperature of PC/KSS/1 mass% PSiN system is lower than that of PC/KSS system, possibly resulting from the thermal depolymerization of PSiN from its chain ends. The end groups of PSiN contain a few hydroxyls, which induce decomposition of the polymer from chain ends in lower temperature [26]. However, the temperature at 50 % of mass loss and the temperature at maximum mass loss rate for the PC/KSS/1 mass% PSiN system are both higher than those of the PC/KSS system. It is speculated that the decomposition of PSiN in lower temperature may be beneficial to cross-linking reactions and formation of a stable structure in PC/KSS/1 mass% PSiN system. In addition, the char residue at 800 °C increases when PSiN is added to PC/KSS. For example, the char residue of the PC/KSS/1 mass% PSiN system is 25.2 %, but 22.9 % for PC/KSS system at the heating rate of 20 °C min−1. This proves that with the addition of PSiN, the thermal stability of PC/KSS/1 mass% PSiN system at high temperature reinforced.
With increasing the heating rate from 5 to 20 °C min−1, the decomposition curves of both PC/KSS and PC/KSS/1 mass% PSiN systems shift to higher temperatures. The activation energies of the thermal degradation of PC/KSS and PC/KSS/1 mass% PSiN systems are determined by the Ozawa method. The linear plots of log r versus 1/T at mass loss values 20, 40, 60, and 70 % are shown in Fig. 6 for PC/KSS and Fig. 7 for PC/KSS/1 mass% PSiN systems, respectively. The calculation results are shown in Table 4. We found that the activation energy of PC/KSS/1 mass% PSiN system is higher than that of PC and PC/KSS system. PSiN increases the thermal degradation activation energy and reduces the decomposition rate of PC. This result is consistent with the results of TG that PC/KSS/1 mass% PSiN system has an higher thermal stability than that of PC/KSS system.
Morphology of the residues’char
The morphology of char residue after LOI tests was further investigated by SEM test, as shown in Fig. 8. From Fig. 8a for PC, there are many holes and cracks on the surface of the residue suggesting a poor char quality. And the structure of char layer for PC/KSS systems are loose and interstitial, as shown in Fig. 8b. This poor char layer cannot be efficiently acted as a barrier to shield the underlying polymer from heat and air. Therefore, in UL-94, the rating of V-0 for PC and PC/KSS system cannot be achieved. However, the char surface of the char for PC/KSS/1 mass% PSiN system illustrated on Fig. 8c is compact, smooth and tight, which can effectively prevent the underlying polymer from the heat and combustible gases and is also less susceptible to crack, in agreement with the improvement of flame retardancy.
Conclusions
A novel flame retardant, containing silicon and nitrogen, was synthesized. It can improve the thermal stabilization and the flame retardancy of polycarbonate (PC). When 3 mass% PSiN is incorporated into PC/KSS system, the LOI value achieves the maximum of 47 and class V-0 of UL-94 test is passed. The thermal degradation activation energy determined by the Ozawa method is increased with the addition of PSiN. TG curves demonstrated that there is a synergistic effect between KSS and PSiN. SEM tests showed that the surface of the char layer for PC/KSS/1 mass% PSiN system is denser and tighter than those of neat PC and PC/KSS system.
References
Li XG, Huang MR. Thermal degradation of bisphenol a polycarbonate by high-resolution thermogravimetry. Polym Int. 1999;48:387–91.
Levchik SV, Weil ED. Overview of recent developments in the flame retardancy of polycarbonates. Polym Int. 2005;54:981–8.
Liu S, Ye H, Zhou Y, He J, Jiang Z, Zhao J, Huang X. Study on flame-retardant mechanism of polycarbonate containing sulfonate-silsesquioxane-fluoro retardants by TGA and FTIR. Polym Degrad Stabil. 2006;91:1808–14.
Zhou W, Yang H, Zhou J. The thermal degradation of bisphenol: a polycarbonate containing methylphenyl-silicone additive. J Anal Appl Pyrol. 2007;78:413–8.
Zaikov GE, Lomakin SM. Polymer flame retardancy: a new approach. J Appl Polym Sci. 1998;68:715–25.
Lu SY, Hamerton I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog Polym Sci. 2002;27:1661–712.
Sen AK, Mukheriee B, Bhattacharya AS, Sanghi LK, De PP, Bhowmick K. Preparation and characterization of low-halogen and nonhalgoen fire-resistant low-smoke (FRLS) cable sheathing compound from blends of functionalized polyolefins and PVC. J Appl Polym Sci. 1991;43:1673–84.
Chiu SH, Wang WK. Dynamic flame retardancy of polypropylene filled with ammonium polyphosphate, pentaerythritol and melamine additives. Polymer. 1998;39:1951–5.
Le Bras M, Bugajny M, Lefebvre J, Bourbigot S. Use of polyurethanes as char-forming agents in polypropylene intumescent formulations. Polym Int. 2000;49:1115–24.
Innes J, et al. Flame retardants for polycarbonate—new and classical solutions. Plast Addit Compd. 2006;8:26–9.
Ishli K, Shimomai K. Flame-retardent polycarbonate resin composition and a molded product. US Patent. 2002; 6342550.
Blackburn KJ, Gallucci RR, Georgiev EM. Flame retardant polycarbonate resin/ABS graft copolymer blends. US Patent. 2003; 6605659.
Chen XL, Hu Y, Song L. Thermal behaviors of a novel UV cured flame retardant coatings containing phosphorus, nitrogen and silicon. Polym Eng Sci. 2008;48:116–23.
Zhong HF, Wei P, Jiang PK. Synthesis and characteristics of a novel silicon-containing flame retardant and its application in poly[2,2-propane-(bisphenol)carbonate]/acrylonitrile butadiene styrene. J Polym Sci B. 2007;45:1542–51.
Masatoshi I, Shin S. Silicone derivatives as new flame retardants for aromatic thermoplastics used in electronic devices. Polym Adv Technol. 1998;9:593–600.
Zhong HF, Wei P, Jiang PK. Thermal degradation behaviors and flame retardancy of PC/ABS with novel silicon-containing flame retardant. Fire Mater. 2007;31:411–23.
Liu SM, Lang XM, Ye H. Preparation and characterization of copolymerized aminopropyl/phenylsilsesquioxane microparticles. Eur Polym J. 2005;41:996–1001.
Nishihara H, Suda Y, Sakuma T. Halogen- and phosphorus-free flame retardant PC plastic with excellent moldability and recyclability. J Fire Sci. 2003;21:451–64.
He QL, Song L, Hu Y, Zhou S. Synergistic effects of polyhedral oligomeric silsesquioxane (POSS) and oligomeric bisphenyl A bis(diphenyl phosphate) (BDP) on thermal and flame retardant properties of polycarbonate. J Mater Sci. 2009;5:1308–16.
Song L, He QL, Hu Y, Chen H, Liu L. Study on thermal degradation and combustion behaviors of PC/POSS hybrids. Polym Degrad Stabil. 2008;93:627–39.
Periadurai T, Vijayakumarb CT, Balasubramanian M. Thermal decomposition and flame retardant behaviour of SiO2-phenolic nanocomposite. J Anal Appl Pyrol. 2010;89:244–9.
Ozawa T. A new method of analyzing thermogravimetric data. Bull Chem Soc Jpn. 1965;38:1881–6.
Polli H, Pontes LAM, Souza MJB, Araujo AS. Thermal analysis kinetics applied to flame retardant polycarbonate. J Therm Anal Calorim. 2006;86:469–73.
Perret Birgit, Kristin H, Pawlowski, Bernhard Schartel. Fire retardancy mechanisms of arylphosphates in polycarbonate (PC) and PC/acrylonitrile-butadiene-styrene. J Therm Anal Calorim. 2009;97:949–58.
Takashi K, John RS, Richard HH. Flame-retardant mechanism of silica: effects of resin molecular weight. J Appl Polym Sci. 2003;87:1541–53.
Grassie N, Francey KF, Macfarlane IG. The thermal degradation of polysiloxanes-part 4: poly(dimethyl/diphenyl siloxane). Polym Degrad Stab. 1980;2:67–83.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yuan, D., Yin, H. & Cai, X. Effect of a novel flame retardant containing silicon and nitrogen on the thermal stability and flame retardancy of polycarbonate. J Therm Anal Calorim 111, 1531–1537 (2013). https://doi.org/10.1007/s10973-012-2488-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2488-y