Abstract
Since administration of capecitabine tablets leading to dose limiting makes the unfavorable toxicity, preparation of sustained-release tablets will overcome most of these side effects. The aim of this study was to prepare and study the stability of capecitabine sustained-release tablets. Sustained-release tablets of capecitabine were characterized by differential scanning calorimetry, X-ray diffraction, and infrared and ultraviolet spectroscopy techniques to determine the stability of the tablets. All tests carried out for tablets upon preparation as well as 6 and 12 months after preparation. The gradual decomposition of capecitabine sustained-release tablets stored at accelerated conditions (40 °C in 75 % of relative humidity) was indicated by decreasing values of peak purity and melting temperature, calculated from the Van’t Hoff equation. Except for the occurrence of one sharp peak for long-term stability and some sharp peaks in the accelerated condition, all peaks showed a crystallized nature. But the FTIR and UV results showed that there were no changes between the initial sustained-release tablets and stored tablets. Although the XRD results showed more peaks in the accelerated condition tablets, the crystalline form of capecitabine was maintained. These findings demonstrate that the capecitabine sustained-release tablet has excellent stability in normal and long-term storage conditions, with slight changes in the accelerated condition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Capecitabine [5′-deoxy-5-fluoro-N-((pentyloxy) carbonyl)-cytidine] is a fluoropyri-midinecarbamate which has antineoplastic activity. This chemical is a prodrug of 5′-deoxy-5-fluorouridine (5′-DFUR), which is enzymatically converted in vivo to 5-fluorouracil (5-FU), and can used in colorectal and breast cancer treatments [1].
Capecitabine absorbs in gastrointestinal tract. In the liver, Capecitabine is hydrolyzed by carboxy esterase to 5′-deoxy-5-fluorocytidine (5′-DFCR). This component changes to 5′-deoxy-5-fluorouridine (5′-DFUR) by cytidinedeaminase, an enzyme found in most tissues. As a subsequence, 5′-DFUR is hydrolyzed by the thymidine phosphorylase enzyme (dThdPase) to 5-FU, an active drug [2–4].
5-FU is metabolized in all cells (normal and tumor cells) into two metabolites: 5-fluoro-2-deoxyuridinemonophosphate (FdUMP) and 5-fluorouridine triphosphate (FUTP). Both metabolites cause cell injury through different mechanisms: the former affects DNA through the binding of FdUMP and folate cofactors, which inhibits the formation of thymidylate from uracil; while the latter affects RNA and protein synthesis by wrongly integrate FUTP instead of UTP [1, 2].
In drug studies, stability of the active ingredient is the most important study. The aim of this research was to find environmental factors and conditions, such as temperature and humidity, which may affect quality of the tablets. Fourier transform infrared spectroscopy (FTIR) is a useful method to detect the occurrence of polymorph transition (hydration and dehydration). To investigate the purity of the active substance, differential scanning calorimetry (DSC) was used. The purpose of this study was to investigate the influence of temperature and humidity on the stability of capecitabine sustained-release tablets.
Experimental methods
Materials
Capecitabine was kindly provided by Osvah Pharmaceutical Company (Tehran, Iran). Capecitabine sustained-release (SR) tablets were prepared (with Carbomer 934p, Hydroxypropyl methylcellulose K4 M, Sodium alginate, Sodium bicarbonate, PEG 3500 and Magnesium Stearate) and used for stability studies according to ICH procedures. SR tablets were stored at the following temperatures and relative humidities (RH): Long term (25 ± 2 °C, 60 % RH ± 5 % RH) and accelerated term (40 ± 2 °C, 75 % RH ± 5 % RH).
To investigate the quality of the finished product and evaluate the drug formulation after different lengths of storage time, and humidity and temperature conditions, stability studies were prepared for 6 and 12 months according to ICH procedures. After the storage times, all samples were analyzed for their physical characteristics. The stability of tablets (n = 4) were studied according to the ICH long term and accelerated procedures. All tablets were stored at standard conditions in incubator (WTB binder APT line) for 6 and 12 months (Table 1).
All tablets were packed in polyethylene bags. The bags were clamped using clamping tape and double packed by placing in a cardboard box with a sealed plywood lid [1, 5–7].
Equipments
The FTIR spectra were recorded on the Perkin-Elmer RX1-FT-IR system. The scanning range was from 4,000 to 400 cm−1. The optimum SR tablets (n = 4) were crushed with KBr to form pellets by applying pressure of 104 N [1, 8].
XRD studies investigated the crystallization of the drug before and after storage [8, 9]. The crystallinity of capecitabine, capecitabine in initial SR tablets, and tablets stored at long-term conditions (25 °C) and at accelerated conditions (40 °C) were evaluated by XRD measurements (D8 Advance X-Ray Diffractometer). Samples (n = 4) were analyzed at ambient temperature over a range of 10°–80° 2θ with a step of 0.02° 2θ, where each step was measured for 1 s.
For spectrophotometry (UV–Visible), all three batches (initial tablet, long, and accelerated tablet) (n = 4) were removed in 50 mL of 0.1 N HCl. Then, 3 mL of each dilution was measured using the UV spectrophotometer’s spectroscan (Shimadzu 1601) [10].
To evaluate the drug content of these three storages, ten tablets of each storages were crushed and suspended in 0.1 N HCl to remove the Capecitabine from the tablets. After 24 h, media were filtrated and measured by a UV spectrophotometer (Shimadzu 1601) at 214 nm [10, 11].
DSC studies were carried out using DSC822e (Mettler Toledo) in the atmosphere of nitrogen (0.1–0.2 bar) to investigate the changes of drug before and after storage [8]. Firstly, the DSC instrument was calibrated using indium as a standard sample, then all samples (n = 4), weighing between 5 and 10 mg, were packed in a small aluminum pan with a pierced lid. All pans were heated from 25 to 140 °C, with a scanning rate of 10 °C min−1 [1, 12].
Results and discussion
FTIR study
The FTIR spectra of the capecitabine in initial tablet and SR tablets stored in long term (25 °C) and in accelerated conditions (40 °C) are shown in Fig. 1. FTIR spectral data were used to confirm the API stability in the initial tablets and stored tablets.
Figure 1 reveals a distinct ether group (C–O) stretching band at 1,050–1,300 cm−1 and an aromatic ring (C–H) band at 750–900 cm−1. The others peaks were assigned as follows: a band appearing at 1,360 cm−1 indicated C–N due to the presence of an amide group; 1,500 cm−1 represented C–C due to the aromatic bond; 1680 cm−1 (sharp) indicated C=C due to an alkene bond; 1,760 cm−1 represented C=O due to a carbonyl bond; 3,010–3,100 cm−1 indicated C–H due to an aromatic ring; 3,300 cm−1 indicated O–H due to phenol or alcohol; and 3,500 cm−1 represented N–H due to amide stretching vibrations. As is shown in Fig. 1, there are no significant changes in FTIR graphs between the initial and stored samples at 25 and 40 °C.
XRD analysis
The XRD technique was performed to evaluate the physical characteristics of the active ingredient. This method is usually used to determine the physical characteristics of drugs and polymers, and can therefore investigate whether a drug is molecularly dispersed in tablet or drug is changed as an ionic interaction. Also, the presence of polymers (indicated by some small sharp peaks) suggests that the polymer structure is crystalline or semi-crystalline. Figure 2 shows that capecitabine morphology in the initial tablet is in the crystalline state [13].
XRD studies of the accelerated and long-term storage condition tablets are shown in Fig. 2. Some changes (sharp peaks) were observed in the accelerated term monograph with that of the initial SR tablets. Some other curves also appeared during conversion to the noncrystalline form. By comparing the monographs of the tablets stored at 25 °C and the initial SR tablets, not many changes were observed during long-term conditions. Thus, it can be concluded that, compared with all figures, the capecitabine is present in the complex has a crystalline character.
Spectrophotometry (UV–visible)
Figure 3 displayed the UV absorption spectra of different samples. There are several main peaks; at 214, 240 nm, and a broad peak at 307 nm.
The capecitabine structure (stability) results (Fig. 3) shows a characteristic shift of the UV absorption peaks to lower/higher energy, or in other words, to higher/lower wavelengths. The comparison between all curves revealed that they were the same and there were no significant changes between the initial and stored samples at 25 and 40 °C, respectively.
Drug content
The drug content test showed the content of capecitabine in initial tablet (99.79 ± 0.48), 40 °C (98.06 ± 0.61) and 25 °C (99.37 ± 0.82).
DSC analysis
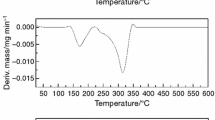
The DSC test was performed to evaluate the influence of storage conditions, such as time, temperature, and humidity, on the stability of capecitabine. The DSC curves of the pure capecitabine as well as the SR tablets and long-term storage tablets stored at 25 °C showed the endotherm coming from the capecitabine melting [1, 13].
Thermal parameters such as melting point (onset) and peak are similar for the SR and long-term tablets (peak = 116, 115 °C), and did not change significantly during storage at 25 °C. However, significant changes were observed during storage at accelerated conditions. Figure 4 shows the DSC curves of the SR tablet after long term and accelerated storage conditions. A decrease in the peak and onset from SR tablets to accelerated storage tablets is observed. For the SR and long-term storage tablets, the peaks were 116 and 115 °C, respectively, while the onset were 108 and 106 °C, respectively. However, peak at 101 °C and onset at 88.41 °C were observed in the accelerated-storage tablet.
Figure 4 shows that the Capecitabine is not stable at 40 °C in 75 % RH. This may suggest that the main factor in the process of capecitabine degradation is the water sorption, which is better at higher temperatures and humidity.
Conclusions
DSC proved the stability of capecitabine studied after 12 months of storage at 25 °C in 60 % RH. It shows capecitabine is not stable at 40 °C in 75 % RH. This may suggest that the main factor in the process of capecitabine degradation is the water sorption, which is better at higher temperature and humidity. In XRD, as the results showed, the structure of capecitabine did not change and the crystallinity of the active ingredient was maintained. Also, there were no significant changes in the FTIR and UV graphs between the initial and stored samples.
Abbreviations
- μg:
-
Microgram
- ACC:
-
Accelerated time
- API:
-
Active pharmaceutical ingredient
- Cap:
-
Capecitabine
- FDA:
-
Food and drug administration
- Long:
-
Long term
- RH:
-
Relative humidity
- USP:
-
United States of pharmacopeia
- UV:
-
Ultraviolet
References
Łaszcz M, Trzcińska K, Filip K, Szyprowska A, Mucha M, Krzeczyński P. Stability studies of capecitabine. J Therm Anal Calorim. 2011;105(3):1015–21.
Walko CM, Lindley C. Capecitabine: a review. Clin Ther. 2005;27(1):23–44. doi:10.1016/j.clinthera.2008.01.00S.
Budman RD. Capecitabine. Invest New Drugs. 2000;18:355–63.
Nutley. Xeloda (capecitabine). Food and Drug Administration (FDA). 2003. http://www.accessdata.fda.gov/drugsatfda_docs/label/2000/20896lbl.pdf.
Ahmad I, Shaikh RH. Effect of temperature and humidity on hardness and friability of packaged paracetamol tablet formulations. Pak J Pharm Sci. 1994;7(2):69–78.
International Conference on Harmonization (ICH). Guidance for Industry Q1A(R2) Stability Testing of New Drug Substances and Products. Food and Drug Administration. 2003. http://www.fda.gov/cber/gdlns/ichstab.pdf.
Russo Karen A. The role of USP monographs in stability testing. In: Huynh-Ba K, editor. Pharmaceutical Stability Testing to Support Global Markets: Pharmasp. Arlington: American Association of Pharmaceutical Scientists; 2010. p. 51–60.
Agnihotri SA, Aminabhavi TM. Novel interpenetrating network chitosan-poly(ethylene oxide-g-acrylamide) hydrogel microspheres for the controlled release of capecitabine. Int J Pharm. 2006;324(2):103–15.
Gong X, Moghaddam MJ, Sagnella SM, Conn CE, Danon SJ, Waddington LJ, et al. Lyotropic liquid crystalline self-assembly material behavior and nanoparticulate dispersions of a phytanyl pro-drug analogue of capecitabine: a chemotherapy agent. ACS Appl Mater Interfaces. 2011;3(5):1552–61. doi:10.1021/am200117u.
Pare A, Yadav SK, Patil UK. Formulation and evaluation of effervescent floating tablet of amlodipine besylate. Res J Pharm Tech. 2008;1(4):526–30.
Pharmacopoeia British. Uniformity of Content. London: Stationery Office on behalf of the (MHRA); 2010.
Chieng N, Rades T, Aaltonen J. An overview of recent studies on the analysis of pharmaceutical polymorphs. J Pharm Biomed Anal. 2011;55(4):618–44.
Dogra Sanjeev. A chitosan–polymer hydrogel bead system for a metformin HCl controlled release oral dosage form. Toledo: BiblioLabsII; 2011.
Acknowledgements
This study was supported by research grants from IPPP, University of Malaya, Malaysia (Grant No.: PS202/2010B). Also, the authors thank Osvah Pharmaceutical Company, Tehran, Iran for their gift of capecitabine and also to Mrs. Fatemeh Allah Bedashti for her friendship and assistance.
Conflict of interest
There is no conflict of interest in this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davoudi, E.T., Noordin, M.I., Javar, H.A. et al. Stability study of the gastric floating dosage form of capecitabine. J Therm Anal Calorim 115, 2495–2499 (2014). https://doi.org/10.1007/s10973-013-3540-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-013-3540-2