Abstract
Differential scanning calorimetry and thermogravimetry techniques were successfully used for stability studies of capecitabine. Decreasing values of melting temperature, heat of fusion, and peak purity calculated from the Van’t Hoff equation indicated the gradual decomposition of capecitabine stored at 40 °C in 75% of relative humidity. The increase in mass loss connected with the water sorption was observed simultaneously. High performance liquid chromatography proved the results of thermoanalytical studies. Infrared spectroscopy (IR) appeared to have the lower sensitivity for the decomposition products detection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Capecitabine ((pentyl[1-(3,4-dihydroxy-5-methyl-tetrahydrofuran-2-yl)-5-fluoro-2-oxo-1H-pyrimidin-4-yl]aminomethanoate)) (Xeloda®, Roche) is an oral carbamate of fluoropyrimidine, a precursor of 5-fluorouracil used in the treatment of metastatic breast and colorectal cancers. Capecitabine is a prodrug, that is enzymatically converted to 5-fluorouracil in the tumor, where it inhibits DNA synthesis and slows the growth of tumor tissue. The activation of capecitabine follows a pathway with three enzymatic steps. The final step of conversion to 5-fluorouracil is catalyzed by thymidine phosphorylase, which is four times more active in tumor cells than in a healthy tissue [1].
Stability testing of an active substance is one of more important steps in drug candidate studies. The purpose of these studies is to answer the question how the quality of a drug substance varies with time under the influence of a variety of environmental factors like temperature and humidity in order to establish a re-test period for a drug substance and recommend storage conditions [2–8].
The most useful technique in a stability testing is high performance liquid chromatography which allows to control an impurities profile arising during a storage. Infrared spectroscopy is a helpful method when a polymorph transition or hydratation/dehydratation processes occur [9–11]. Among these techniques differential scanning calorimetry is a complementary method when purity is considered. The application of the modified Van’t Hoff equation which is based on the fact that eutectic impurities depress a melting point of an examined sample has been discussed in details [12–14]. The main advantages of thermoanalytical methods are a minimal sample amount, a short time of analysis in comparison with chromatographic one and the lack of reference standards [15–18].
The aim of this article is to investigate the influence of storage conditions such as time, temperature, and humidity on stability of capecitabine. The purity of capecitabine is studied by means of scanning differential calorimetry and compared to chromatographic results.
Experimental
Materials
Two samples of capecitabine: KP-1 and KP-2, produced at the Pharmaceutical Research Institute in Warsaw and the commercial one KP-API2 (Aurisco Pharmaceutical Limited Batch No. 081105) were taken into the stability studies. Capecitabine was stored at following temperature and relative humidity (RH) conditions: 25 ± 2 °C, 60 ± 5% RH (climate chamber Binder KBF 720) and 40 ± 2 °C, 75 ± 5% RH (climate chamber Binder KBF 240). Samples were packed into polyethylene bags. The bags were closed with a clamping tape and inserted into cardboard drums with a plywood lid. The lid was fastened with the use of a clamping ring, which was then sealed. Samples KP-2 and KP-API2 were stored at the temperature of 25 °C in the humidity of 60% for 6 months (KP-2a and KP-API2a). Samples KP-1 and KP-API2 were stored at 40 °C in 75% and studied after following time periods: 1 month (KP-1a and KP-API2b), 2 months (KP-1b and KP-API2c), 3 months (KP-1c and KP-API2d), and 6 months (KP-1d and KP-API2e).
Thermal analysis (DSC and TGA)
Thermal analyses were carried out by means of the DSC822 with IntraCooler and the TGA/SDTA 851 cells (Mettler Toledo) in the nitrogen atmosphere. Accurately weighed samples (5–7 mg) were packed in the aluminum pan with the pierced lid. For DSC experiments, samples were heated from 25 to 140 °C, with the scanning rate of 10 °C/min. For TGA experiments, samples were heated from 25 to 180 °C, with the scanning rate of 5 °C/min. The DSC instrument was calibrated using indium and zinc as standards, whereas for the TGA instrument indium and aluminum were used. TGA measurements were blank curve corrected.
High performance liquid chromatography (HPLC)
The HPLC system consisted of a Shimadzu LC 20A separations module with a SPD-M20A detector set at 250 nm. The chromatographic separations were performed using a Luna C18 analytical column (4.6 × 250 mm, 5 μm particle size; Phenomenex). The column temperature was kept at 39 °C and the samples temperature at 15 °C. The flow rate was maintained at 1 mL/min. The mobile phase A and B consisted of 0.1% acetic acid in water:methanol:acetonitrile (600:350:50 and 150:800:50, v/v/v, respectively). Gradient parameters are given in Table 1. The samples were dissolved and diluted in the mixture of water:methanol:acetonitrile, 60:35:5, v/v/v; the concentration was about 0.6 mg/mL. The injection volume was 10 μL.
Water content determination
Karl Fischer volumetric titration was used to determine water content in capecitabine.
Infrared spectroscopy (IR)
The IR spectra were recorded on the Perkin-Elmer FT-IR BX spectrometer in the range from 4000 to 400 cm−1. A spectral resolution of 4 cm−1 was used. Solid samples were measured in KBr pellets.
Results and discussion
DSC curves of the initial samples KP-2 and KP-API2 as well as samples KP-2a and KP-API2a stored at 25 °C show the single endotherm coming from the substance melting. Thermal parameters such as heat of fusion and melting point (onset) are similar for the initial and stored samples (ΔH ≈ 80 J/g and onset ≈ 120 °C) and do not change significantly during the storage. Also the peak purity evaluated for these samples is not lower than 99 mol%. After storage, the mass loss evaluated to 112 °C, also does not change substantially. For the sample KP-2a the mass loss increases twice form the initial value of 0.10% (KP-2), for the sample KP-API2 is at the similar level of 0.22%. For the initial and stored samples the water content was determined by Karl Fischer method in order to calculate the part of water in the overall mass loss. The water content for samples KP-2 and KP-2a is at the similar level of 0.02%, for samples KP-API2 and KP-API2a the water content also does not change. The remaining part of the mass loss comes from residual solvents used during the crystallization process. Thermal analysis proved the stability of capecitabine studied after 6 months of storage at 25 °C in 60% RH. The purity determined by means of the HPLC method is comparable to the peak purity calculations by DSC (Table 2).
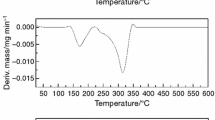
Significant changes are observed during stability studies at 40 °C in 75% RH. Figures 1 and 2 show DSC curves of the initial samples KP-1 and KP-API2, respectively, in the comparison with curves of these samples measured after 1, 2, 3, and 6 months of the storage at 40 °C. In this case the decrease in heat of fusion and melting point is observed. For the sample KP-1 ΔH and onset values decrease from 80.85 J/g and 119.61 °C to 70.47 J/g and 116.60 °C after 6 months of storage. The DSC method of the purity determination proved the degradation of capecitabine. For the initial sample KP-1 the purity decreases from 99.41 mol to 96.94 mol% for the sample stored 6 months. For the initial sample KP-API2 the purity decreases from 99.30 to 96.02 mol% (after 6 months of storage). HPLC analyses of KP-1 and KP-API2 also proved a decomposition during the storage at 40 °C. For the sample KP-1 the HPLC purity decreases from 99.98 to 97.73% after 6 months of storage. For the sample KP-API2 the purity decreases from 99.97 to 97.88% after 6 months of storage (Table 3). The HPLC analysis of the KP-API2 and KP-1 samples after 6 months in 40 °C revealed the presence of two main impurities at RRT of 0.15 and 0.17, apart from the capecitabine peak at RT of 22.5 min (RRT 1.00). Based on the literature data [19–21] as well as degradation studies of capecitabine done in Pharmaceutical Research Institute, the impurities were identified as 5′-deoxy-5-fluorocytidine (USP Related Compound A) and 5′-deoxy-5-fluorouridine (USP Related Compound B), respectively, and confirmed by the co-injection of authentic samples (USP Reference Standards). The structures of the afore-mentioned impurities are shown in Fig. 3 and the representative chromatograms generated to confirm the sameness of examined compounds are shown in Fig. 4.
Also the mass loss is changed (Fig. 5). For samples KP-1 and KP-API2 the mass loss increases from the initial value of 0.12 to 0.39% (after 6 months of storage) and from 0.10 to 0.53% (after 6 months of storage), respectively. After storage, in both samples, the water content increases eight times from initial value of 0.02%. The water content measured for stored samples is smaller than the mass loss which can suggest the partial decomposition of capecitabine under influence of water sorption.
There are no visible changes in IR spectra between the initial and stored samples at 25 and 40 °C (Fig. 6).
Conclusions
The studies demonstrated that the DSC method is a powerful tool in a purity analysis. By means of thermoanalytical techniques as well as the HPLC method the stability of capecitabine after 6 months of storage at 25 °C in 60% RH was proved. The substance was not stable at 40 °C in 75% RH. It was proved that the main factor in the process of capecitabine degradation is the water sorption which is privileged at higher temperature and humidity.
References
Stec R, Bodnar L, Szczylik C. Capecitabine in palliative chemotherapy of colorectal cancer–efficacy and toxicity. Contemp Oncol. 2009;13:167–76.
ICH, Q 1 A (R2) guideline, August 2003, CPMP/ICH/2736/99.
Lizarraga E, Zabaleta C, Palop JA. Thermal stability and decomposition of pharmaceutical compounds. J Therm Anal Calorim. 2007;89:783–92.
Rezende RLO, Santoro MIRM, Matos JR. Stability and compatibility study on enalapril maleate using thermoanalytical techniques. J Therm Anal Calorim. 2008;93:881–6.
Michalik K, Drzazga Z, Michnik A, Kaszuba M. Thermal stability study of the protease inhibitors Nelfinavir mesylate and atazanavir sulfate. J Therm Anal Calorim. 2007;88:401–4.
Howell BA, Chhetri P, Dumitrascu A, Stanton KN. Thermal degradation of platinum(IV) precursors to antitumor drugs. Therm Anal Calorim. 2010;102:499–503.
Mocanu AM, Odochian L, Apostolescu N, Moldoveanu C. Comparative study on thermal degradation of some new diazoaminoderivatives under air and nitrogen atmospheres. J Therm Anal Calorim. 2010. doi:10.1007/s10973-010-0857-y.
Cheng X-X, Lui Y, Hu Y-J, Liu Y, Li L-W, Di Y-Y, Xiao X-H. Thermal behavior and thermodynamic properties of berberine hydrochloride. J Therm Anal Calorim. 2009. doi:10.1007/s10973-009-0288-9.
Ławecka M, Kosmacińska B, Glice M, Korczak K. The influence of storage conditions on the polymorphic stability of zolpidem tartrate hydrate. J Therm Anal Calorim. 2006;83:583–5.
Kojima T, Yamauchi Y, Onoue S, Tsuda Y. Evaluation of hydrate formation of a pharmaceutical solid by using diffuse reflectance infrared Fourier-transform spectroscopy. J Pharm Biomed Anal. 2008;46:788–91.
Plano D, Lizarraga E, Palop JA, Sanmartín C. Study of polymorphism of organosulfur and organoselenium compounds. J Therm Anal. 2010. doi:10.1007/s10973-010-1012-5.
Giron D, GoMbronn C. Place of DSC purity analysis in pharmaceutical development. J Therm Anal. 1995;44:217–51.
Yamamoto K, Momota M, Katayama S, Narita K. Determination of the purity of multi-component organic substances by DSC. Anal Sci. 1998;14:599–602.
Faroongsarng D, Kadejinda W, Sunthornpit A. Thermal behaviour of a pharmaceutical solid acetaminophen doped with p-aminophenol. AAPS PharmSciTech. 2000;1(3) article 23.
Swarbrick J. Encyclopedia of pharmaceutical technology. vol 6. 3rd ed. New York: Informa Healthcare; 2006.
The actual edition of USP Pharmacopeia, chapter 891.
Thermal Analysis UserCom 10, Mettler-Toledo, Inc, 1900 Polaris parkway, Columbus; 43240.
Mathkar S, Kumar S, Bystola A, Olawoorea K, Mina D, Markovicha R, Rustuma A. The use of differential scanning calorimetry for the purity verification of pharmaceutical reference standards. J Pharm Biomed Anal. 2009;49:627–31.
Salvador A, Millerioux L, Renou A. Simultaneous LC-MS-MS analysis of capecitabine and its metabolites (5′-deoxy-5-fluorocytidine, 5′-deoxy-5-fluorouridine, 5-fluorouracil) after off-line SPE from human plasma. Chromatographia. 2006;63:609–15.
Xu Y, Grem JL. Liquid chromatography–mass spectrometry method for the analysis of the anti-cancer agent capecitabine and its nucleoside metabolites in human plasma. J Chromatogr B. 2003;783:273–85.
The actual edition of USP Pharmacopeia. Capecitabine; 1774.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Łaszcz, M., Trzcińska, K., Filip, K. et al. Stability studies of capecitabine. J Therm Anal Calorim 105, 1015–1021 (2011). https://doi.org/10.1007/s10973-011-1351-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1351-x