Abstract
The aim of this research was to analyse composition, fatty acids distribution and oxidative stability of fats extracted from four samples of baby formulas. The fats were oxidized in a differential scanning calorimeter (DSC) under polythermal (dynamic) conditions and at normal pressure. The DSC experiments were carried out in an oxygen flow atmosphere using different, linearly programmed, heating rates in the range of 4–12.5 °C/min. The extrapolated onset temperatures were determined using DSC exotherms and used for the assessment of the thermal oxidative stabilities of the samples. Activation energies (E a), pre-exponential factors (Z) and reaction rate constants (k) for oil oxidation under DSC conditions were calculated using the Ozawa–Flynn–Wall method and the Arrhenius equation. The melting characteristics of the studied fats were obtained. The fats extracted from the agglomerated samples with higher onset temperatures were more stable than the fats extracted from the initial samples of baby formulas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Milk is spray dried for easier storage, handling and transport. The spray drying process involves atomising the milk within a flow of hot air, where water is progressively evaporated from the droplets until dried milk particles are produced. The functional properties of the resultant milk powder, such as particle size distribution, bulk density, flowability and solubility, determine its storage, handling and transport capabilities [1]. Milk powders with a standard fat content of 26 g per 100 g of milk are usually traded commercially for a variety of dairy and food application end-uses [2]. The major ingredient in milk powder is lactose [3]. The presence of other components such as moisture, protein(s), fat, mineral and lactic acid can largely affect the physicochemical characteristics including water absorption, glass transition temperature and crystallization of all milk powders [4]. Dietary lipids are the major energy source for infants and young children [5, 6]. The most important functional components of dietary lipids, triglycerides, phospholipids and cholesterol esters and particularly long-chain polyunsaturated fatty acids (LC-PUFA), are fundamental to normal growth and development of infants [7]. The two families of LC-PUFAs, the n-3 and the n-6, have specific functions: docosahexaenoic acid (DHA; C22:6n3) in retina and brain, whereas arachidonic acid (AA; C20:4n6) is the precursor of prostaglandins and leucotrienes and is also a major brain component [6, 8]. Long-chain omega-3 PUFAs have a well-established role in lowering blood serum triacylglycerol and cholesterol concentrations [9]. On the other hand, PUFAs, such as fish oil and flax seed oil, are very susceptible to oxidation during processing and storage resulting in decreased nutritional value and sensory quality. One possible way to protect lipids against oxidation is encapsulation [10]. Encapsulation of active food ingredients is an important application that can be attained by nanotechnological approaches [11], for example, in the case of long-chain fatty acids.
The benchmark for any form of lipid-based product is the oxidative stability or resistance to oxidation [12]. Lipid oxidation is a free radical chain reaction that leads to the development of unpleasant taste and undesirable changes such as the formation of off-flavour compounds (aldehydes, ketones, etc.) [13]. Oxidation reactions consist of an initiation, a propagation and a termination stage. Several methods have been used to analyse and monitor lipid oxidation [14, 15]. These reactions release heat which can be measured using differential scanning calorimetry (DSC). Recording the heat released from a particular reaction using DSC can be conducted in either isothermal or non-isothermal mode. In general, non-isothermal methods are widely used in lipid oxidation because they can provide valuable analytical and kinetic information. In addition, the DSC method is simple, convenient and fast [16, 17]. The non-isothermal method is based on the linear correlation between the temperature that corresponds to a specific thermal event and a different heating rate [18]. From the relationship that follows, an Arrhenius type equation, the effective activation energy (E a), pre-exponential factor (Z), and constant rate (k) are derived [15]. DSC has long been a useful tool to obtain the melting characteristics of edible fats and oils [19–21]. Their measurement by DSC is a key indicator of changes in their structure. DSC is able both to impose a thermal history and to measure the rate of melting as a function of temperature [22].
The aim of this article was to assess the oxidative stability of fats extracted from powdered baby formulas (mixtures, agglomerates).
Materials and methods
Materials
The powders used in this study were: skimmed and whole milk powder produced by District Dairy Cooperative in Koło, Poland, essential PUFAs Ropufa “10” n-3 Food Powder S/SD and Ropufa “10” n-6 Food Powder distributed by DSM Nutritional Products Co. Ltd in Mszczonów, Poland and lactose powder distributed by Hortimex Co. Ltd in Konin, Poland. Milk fat content in the skimmed milk powder was 0.5% and in whole milk powder—26%. Lactose was an ingredient of skimmed and whole milk powders and its content in both powders was 49.5%. A 15% lactose solution was used as a wetting liquid in agglomeration process. The compositions of mixtures and agglomerates are shown in Table 1. M-1 and M-2 represent powder mixtures; A-1 and A-2 represent agglomerated samples.
Agglomeration
The technological processes involved in the production of powdered mixtures with n-3 and n-6 were: mixing, wet agglomeration and drying. These processes were carried out in a fluidized bed agglomerator of the STREA 1 type produced by Niro-Aeromatic A.G., Bubendrof, Switzerland. The wet agglomeration process in the fluidized bed used 15% lactose solution as the wetting liquid. A sample of food powder weighing 300 g was placed in the product container, and fluidized by means of an upward flowing air stream. The temperature of the inlet fluidizing air entering the bed was set at 50 °C. During agglomeration, it was necessary to increase fluidizing air flow regularly in order to maintain correct fluidization of enlarged agglomerates. When the binder solution had been used up, the product was dried for 15 min at 50 °C [23, 24].
Extraction of the lipid fraction
The samples were homogenized with chloroform/methanol (2/1). Next, the homogenates were washed with 0.9% NaCl solution. After vortexing for some seconds, the mixtures were centrifuged at low speed (2,000 rpm) to separate the two phases. After centrifugation and syphoning of the upper phase, the lower chloroform phase containing lipids was evaporated under vacuum in a rotary evaporator.
Determination of fatty acid composition
The determination of fatty acid composition was carried out by gas chromatographic (GC) analysis of fatty acid methyl esters. A Shimadzu GC 17A chromatograph equipped with a flame ionization detector and a BPX-70 capillary column of 0.22 mm (internal diameter) × 30 m length and 0.25 mm film thickness was used. The oven temperature was programmed as follows: 60 °C for 1 min, then it was increased by 10 °C/min to 170 °C; from 170 to 230 °C, it was increased by 3 °C/min; then kept at 230 °C for another 15 min. The temperature of the split injector was 225 °C, with a split ratio of 1:100, and the detector temperature was 250 °C. Nitrogen flowing with the rate of 1 ml/min was used as the carrier gas. The identification of fatty acids was carried out using the lipid standard purchased from Sigma Aldrich, Supelco Analytical, Bellefonte, PA, USA [25].
DSC measurements
The calorimetric measurements were performed with a Q200 DSC (TA Instruments, New Castle, DE, USA). Oxygen was used as the purge gas at a rate of 50 ml/min. The instrument was calibrated in temperature and enthalpy with high purity indium according to the procedure for standard DSC. A normal-pressure DSC cell was used. Fat samples of 3–4 mg were placed in aluminium sample pans and inserted into the heating chamber of the DSC cell. The aluminium reference pan was left empty. Samples of compounds were heated in an open aluminium pan with linear heating rates of 4, 6, 7.5, 10, and 12.5 °C/min. For each programmed heating rate (β, °C/min), at least triplicate determinations were carried out. Each run was recorded on the instrument’s computer disc. When the run was completed, the onset oxidation temperature (T on, °C) was determined as the intersection of the extrapolated baseline and the tangent line (leading edge) of the recorded exotherm. The averages from measurements of t ON for each fat at a given temperature were determined as the intersection of the extrapolated baseline and the tangent line (leading edge) of the recorded exotherm [25–28].
Melting characteristics
DSC measurements of melting characteristics were carried out with a Q200 DSC (TA Instruments, New Castle, DE, USA). Calibration was done with indium standards. Samples of 3–4 mg were placed into aluminium pans with a lid and were non-hermetically sealed. An empty sealed aluminium pan was used as a reference and the experiments were performed under a nitrogen flow rate of 50 ml/min, normal pressure. Melted samples were heated to 80 °C and held for 10 min, in order to melt all the crystals and to erase the thermal memory. The samples were then cooled to −80 °C at 10 °C/min and maintained at −80 °C for 30 min. Then the melting (so-called second fusion) profiles were obtained by heating the samples to 80 °C at a heating rate of 15 °C/min [29, 30].
Integration and onset temperature measurements were performed using the functions of the Universal Analysis Software (TA Instruments).
Statistical analysis
Each analysis was carried out in triplicate. The data were reported as the means ± standard deviation. Two-way ANOVA was performed using the Statgraphics Plus for Windows, version 4.1 (Statistical Graphics Corporation, Warrenton, VA, USA). Differences were considered to be significant at a p value of 0.05, according to Tukey’s multiple range test.
Results and discussion
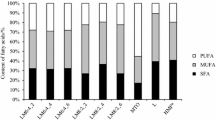
Milk fat is a complex mixture of triacylglycerols, composed of a large number of different fatty acids which leads to a heterogeneous composition and a very broad melting range varying between −40 and 40 °C [31]. The fatty acid composition of the fat extracted from powdered baby formulas enriched in n-3 and n-6 fatty acids are presented in Table 2.
The saturated fatty acids range is 45.5–55%, the monounsaturated fatty acids 22.3–25.5%, and the polyunsaturated 19.932%. Low levels of trans-isomers were detected (0.2–1.5%). The powdered baby formulas contained mainly palmitic acid (19.6–31.30%) and oleic acid (20.0–22.5%). The addition of the n-3 and n-6 fatty acids increased the unsaturated fatty acids content. The content of DHA was 2.3% in the agglomerated baby formulas obtained from skimmed milk powder and 3.2% in the agglomerated baby formulas obtained from skimmed and whole milk powders. DHA, a n-3 fatty acid, is an integral component of cell membranes in the developing brain and retina; and it is one of the essential fatty acids for infant development. DHA in infant formulas influences the cognitive ability, motor ability, visual acuity and visual maturation of the infant [32]. Newborn babies are able to synthesize DHA from α-linolenic acid. However, the amount of DHA synthesized in the newborn may be inadequate to meet the requirements of the developing infant [33]. In the past, commercially available infant formulas were often prepared with plant oils containing only 18-carbon essential fatty acids [32]. Milk supplementation in DHA acid is indicated, which in recent times has become increasingly common in infant formulas [34].
The fatty acid composition is an important and most basic parameter for determining melting characteristics of milk fat. The melting characteristics of the fat fraction were studied by means of DSC. DSC provides a generalized view of the melting of fat fraction over its melting range (melting profile). Figure 1 shows the melting profiles of the fat fractions extracted from the agglomerated baby formulas and powder mixtures.
The melting thermograms of the fat from the agglomerated baby formulas were very similar to those of the corresponding fat from the powder mixtures. All exhibited a wide melting range from −80 to 80 °C and two distinct peaks. On the thermograms of the formulas obtained from whole milk and skimmed milk, there was a broad shoulder representing high-melting triacylglycerol species and one peak representing middle-melting triacylglycerol species [30]. Baby formulas (agglomerated and powder mixtures) containing skimmed milk powder have lower melting points than formulas with skimmed and whole milk powder. The saturated and unsaturated fatty acid content of fat affects its melting characteristics. Increases in long-chain unsaturated fatty acids (mainly C18:2) with concurrent decreases in long-chain saturated fatty acids (C16:0) resulted in the fat from formulas M-2 and A-2 with lowered melting points and a greater proportion of low-melting triacylglycerol species. Conversely, increases in long-chain saturated fatty acids with concurrent decreases in long-chain unsaturated fatty acids resulted in the fat from formulas M-1 and A-1 with higher melting points and a greater proportion of high-melting triacylglycerol species [32]. These observations indicate that all the analysed formulas (agglomerated and powder mixtures) are complex mixtures of various triacylglycerols with wide-ranging melting points.
Table 3 shows the experimental onset oxidation temperatures (T on) obtained at five heating rates (4, 6, 7.5, 10, 12.5 °C/min). As the heating rate increases, the T on increases. This can be described by the following linear correlation (Eq. 1):
where β is the heating rate, a and b are adjustable coefficients and T on is the onset oxidation temperature.
During slow heating, primary oxidation products such as hydroperoxides, generated during the initial oxidation stage, react with excess of oxygen to form low molecular weight compounds such as aldehydes and acids that remain in solution, accelerating the degradation process. At fast heating rates, on the other hand, these intermediate products are lost through evaporation before they can further react with the fat solution, shifting the start of the DSC peak to higher values [15]. The T on values obtained for each baby formula can be used as primary parameters for the assessment of the resistance of tested fats to its thermal oxidative decomposition [35]. The oxidative stability of the formulas can be ranked based on the rule “the higher onset temperature the more stable the formula”. Both of the agglomerated baby formulas with the highest T on, are more stable than the non-agglomerated baby formulas, whereas the T on values obtained at the same heating rates are lower. Martinez-Monteagudo et al. [15] studied the non-isothermal oxidation of anhydrous milk fat rich in conjugated linoleic acid and obtained much higher onset oxidation temperatures at similar heating rates. These can be attributed to the different ratio between unsaturated and saturated fatty acids. In our experiment, fat was extracted from milk powders enriched in n-3 and n-6 fatty acids and consequently we noted a higher content of unsaturated fatty acids and lower onset temperatures.
In the DSC method which is based on the recording of released heat in non-isothermal mode, the consumption of oxygen can be neglected due to the large excess of oxygen generated by a constant flow rate.
It means that the auto-oxidation is a first order reaction [35]. This fact can be used for the calculation of kinetic parameters such as activation energy (E a), pre-exponential factor (Z) and reaction rate constants (k). Using the information presented in Table 4 and according to Eqs. 2–4:
where R is the gas constant, β is the heating rate (°C/min) and T is temperature (K), the kinetic parameters were calculated and the values are presented in Table 3. For the agglomerated samples, the values of activation energy were 82.91 and 85.69 kJ/mol for the formulas containing whole and skimmed milk powder and the formulas containing skimmed milk powder only, respectively. For the powder mixtures, the values of activation energy were 106.48 and 104.55 kJ/mol for the formulas containing whole and skimmed milk powder and the formulas containing skimmed milk powder only, respectively. The thermal and thermo-oxidative properties of fats are related to the fatty acid composition [36]. The total content of unsaturated fatty acids was 44.8% in M-1 and 46.6% in M-2 powder mixtures. The powder mixture M-1 with lower n-3 and n-6 fatty acids content (19.9%) was characterized by higher activation energy than powder mixture M-2 where content of n-3 and n-6 fatty acids was higher (24.3%). High values of E a of fats present in baby formulas can be due to the high content of saturated fatty acids. Khan et al. [37] concluded that the fats and oils having greater proportion of saturated fatty acids and PUFAs show higher stability under thermal stress. Oleic and linoleic acids in particular influence the thermo-oxidative properties of studied fats, showing similarity with the results presented by López-Beceiro et al. [36]. High linoleic content decreases the Ea for oxidation, and high oleic content increases it [38]. In the case of M-1 with 21.3% of oleic acid and 10.7% of linoleic acid, the activation energy was higher than for M-2 containing 20.8% of oleic acid and 12.4% of linoleic acid. The similar relationships were observed for agglomerated samples A-1 and A-2. A-2, characterized by higher activation energy, contained 22.5% of oleic acid and 8.1% of linoleic acid, whereas sample A-1 had lower activation energy resulting from lower oleic acid and higher linoleic acid content. The activation energies obtained in this article for the thermo-oxidative decomposition of fats fit in with the data given in literature [15, 28, 39, 40]. Martinez-Monteagudo et al. [15] calculated the activation energy in the range 82.42–114.11 kJ/mol for milk fat rich in conjugated linoleic acid, Ostrowska-Ligęza et al. [28] 72–104.3 kJ/mol for olive oil, Kasprzycka-Guttman et al. [39] 62.2 kJ/mol for peanut oil, Arora et al. [40] 71.3–113.4 kJ/mol for soybean oil and 55.4–156.9 kJ/mol for sunflower oil. Activation energy should not be used as the only parameter for comparison of the oxidation stability of different lipid systems.
Conclusions
For the first time, non-isothermal oxidation of fat from agglomerated powders was studied. The kinetic information can help in the assessment of oxidative stability of agglomerated powders-based products. The fats extracted from the agglomerated samples A-1 and A-2 with higher onset temperatures were more stable than the fats extracted from the initial baby formulas (M-1 and M-2) where onset temperatures obtained at the same heating rate were lower. The melting characteristics of the fats from the agglomerates were very similar to their corresponding mixtures.
References
Nijdam JJ, Langrish TAG. The effect of surface composition on the functional properties of milk powders. J Food Eng. 2006;77:919–25.
Keogh M, Murray C, O’Kennedy B. Effects of ultrafiltration of whole milk on some properties of spray-dried milk powders. Int Dairy J. 2003;13:995–1002.
Haque MK, Roos YH. Water plasticization and crystallization of lactose in spray-dried lactose/protein mixtures. J Food Sci. 2004;69:23–9.
Shrestha AK, Howes T, Adhikari BP, Bhandari BR. Water sorption and glass transition properties of spray dried lactose hydrolysed skim milk powder. LWT. 2007;40:1593–600.
Koletzko B. Response to and range of acceptable fat intakes in infants and children. Eur J Clin Nutr. 1999;53(Suppl 1):78–3.
Elmadfa I, Majchrzak D. Fatty acid profile in baby food products. Eur J Lipid Sci Technol. 2000; 2000:270–5.
Clandinin MT. Brain development and assessing the supply of polyunsaturated fatty acid. Lipids. 1999;34:131–7.
Koletzko B, Demmelmair H, Hartl W, Kindermann A, Koletzko S, Sauerwald T, Szitanyi P. The use of stable isotope techniques for nutritional and metabolic research in paediatrics. Early Hum Dev. 1998;53:77–97.
Harris WS, Kris-Etherton PM, Harris KA. Intakes of long-chain omega-3 fatty acid associated with reduced risk for death from coronary heart disease in healthy adults. Curr Atheroscler Rep. 2008;10(6):503–9.
Gökmen V, Mogol BA, Lumaga RB, Fogliano V, Kaplun Z, Shimoni E. Development of functional bread containing nanoencapsulated omega-3 fatty acids. J Food Eng. 2011;105:585–91.
Baeumner A. Nanosensors identify pathogens in food. Food Technol. 2004;58(8):51–5.
Velasco J, Andersen ML, Skibsted LH. Evaluation of oxidative stability of vegetable oils by monitoring the tendency to radical formation. A comparison of electron spin resonance spectroscopy with the Rancimat method and differential scanning calorimetry. Food Chem. 2004;85(4):623–32.
Simon P, Kolman L. DSC study of oxidation induction periods. J Therm Anal Calorim. 2001;64(2):813–20.
Kamal-Eldin A, Pokorny J. Lipid oxidation products and methods used for their analysis. In: Kamal-Eldin A, Pokorny J, editors. Analysis of lipid oxidation. Champaign: AOCS; 2005.
Martınez-Monteagudo SI, Saldana MD, Kennelly JJ. Kinetics of non-isothermal oxidation of anhydrous milk fat rich in conjugated linoleic acid using differential scanning calorimetry. J Therm Anal Calorim. 2011. doi:10.1007/s10973-011-1649-8.
Adhvaryu A, Erhan SZ, Liu ZS, Perez JM. Oxidation kinetic studies of oils derived from unmodified and genetically modified vegetables using pressurized differential scanning calorimetry and nuclear magnetic resonance spectroscopy. Thermochim Acta. 2000;364(1–2):87–97.
Agrawal RK. Analysis of nonisothermal reaction-kinetics. 1. Simple reactions. Thermochim Acta. 1992;203:93–110.
Ozawa T. Critical investigation of methods for kinetic-analysis of thermoanalytical data. J Therm Anal. 1975;7(3):601–17.
Cassel RB. Determining percent solid in an edible fat. New Castle: TA Instruments, Inc.; 2002.
Lopez C, Lesieur P, Keller G, Ollivon M. Thermal and structural behaviour of milk fat, 1 unstable species of cream. J Colloid Interface Sci. 2000;229:62–71.
Lopez C, Briard-Bion V, Camier B, Gassi J-Y. Milk fat thermal properties and solid fat content in emmental cheese: a differential scanning calorimetry study. J Dairy Sci. 2006;89:2894–910.
Hu J, Sari O, Eicher S, Rakotozanakajy AR. Determination of specific heat of milk at different fat content between 1 °C and 59 °C using micro DSC. J Food Eng. 2009;90:395–9.
Kowalska J, Lenart A. The influence of ingredients distribution on properties of agglomerated cocoa products. J Food Eng. 2005;68:155–61.
Jinapong N, Suphantharika M, Jamnong P. Production of instant soymilk powders by ultrafiltration, spray drying and fluidized bed agglomeration. J Food Eng. 2008;84:194–205.
Wirkowska M, Górska A, Bryś J, Ostrowska-Ligęza E, Koczoń P. Oxidative stability and triacylglycerols structure of lipid fraction from cookies for infants. Int J Food Sci Nut. 2011. doi:10.3109/09637486.2011.627838.
Ferrari C, Angiuli M, Tombari E, Righetti MC, Matteoli E, Salvetti G. Promoting calorimetry for olive oil authentication. Thermochim Acta. 2007;459:58–63.
Litwinienko G, Daniluk A, Kasprzycka-Guttman T. Study on autoxidation kinetics of fats by differential scanning calorimetry. 1. Saturated C12–C18 fatty acids and their esters. Ind Eng Chem Res. 2000;39:7–12.
Ostrowska-Ligęza E, Bekas W, Kowalska D, Łobacz M, Wroniak M, Kowalski B. Kinetics of commercial olive oil oxidation: dynamic differential scanning calorimetry and Rancimat studies. Eur J Lipid Sci Technol. 2010;112:268–74.
Danthine S, Deroanne C. Influence of SFC, microstructure and polymorphism on texture (hardness) of binary blends of fats involved in the preparation of industrial shortenings. Food Res Int. 2004;37:941–8.
Aguedo M, Giet JM, Hanon E, Lognay G, Wathelet B, Destain J, Brasseur R, Vandenbol M, Danthine S, Blecker C, Wathelet JP. Calorimetric study of milk fat/rapeseed oil blends and their interesterification products. Eur J Lipid Sci Technol. 2009;111:376–85.
Kim EHJ, Chen XD, Pearce D. Melting characteristics of fat present on the surface of industrial spray-dried dairy powders. Colloids Surf B. 2005;42:1–8.
Wan Y, Bechtel PJ, Sathivel S. Physical and nutritional properties of baby food containing menhaden oil (Breoortia tyrranus) and microencapsulated menhaden oil. Food Sci Technol. 2011;44:576–81.
Koletzko B, Sauerwald U, Keicher U, Saule H, Wawatschek S, Böhles H, Bervoets K, Fleith M, Crozier-Willi G. Fatty acid profiles, antioxidant status, and growth of preterm infants fed diet without or with long-chain polyunsaturated fatty acids. Eur J Nutr. 2003;42:243–53.
Makrides M, Gibson RA, Udell T, Ried K. The International LC-PUFA investigators: supplementation of infant formula with long-chain polyunsaturated fatty acids does not influence the growth of term infants. Am J Clin Nutr. 2005;81:1094–101.
Kowalski B. Thermoanalytical oxidation of edible oils and fats. I. Kinetics of thermal-oxidative decomposition of rapeseed oil. Acta Aliment Pol. 1988;14:195–206.
López-Beceiro J, Artiaga R, Gracia C, Tarrío-Saavedra J, Naya S, Mier JL. Comparison of olive, corn, soybean and sunflower oils by PDSC. J Therm Anal Calorim. 2011;104:169–75.
Khan MN, Sarwar A, Wahab MF. Chemometric assessment of thermal oxidation of some edible oils. J Therm Anal Calorim. 2010;102:369–74.
Erhan SZ. Adhvaryu A. Vegetable based base stocks. In: Erhan SZ, Perez JM, editors. Biobased industrial fluids and lubricants. Champaign: AOCS; 2002. p. 1–19.
Kasprzycka-Guttman T, Jarosz-Jarszewska M, Litwinienko G. Specific heats and kinetic parameters of thermo-oxidative decomposition of peanut oil. Thermochim Acta. 1995;250:197–205.
Arora S, Bagoria R, Kumar M. Effect of alpha-tocopherol (vitamin E) on the thermal degradation behavior of edible oils. Multiple-heating rate kinetics. J Therm Anal Calorim. 2010;102:375–81.
Acknowledgements
This study was supported by the Ministry of Science and Higher Education Grant No. NN312 366637. The results of this research were presented at the CEEC-TAC1 Conference. The authors express their gratitude to Mr Andrzej Kubiszyn for editing and proofreading of this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wirkowska, M., Ostrowska-Ligęza, E., Górska, A. et al. Thermal properties of fats extracted from powdered baby formulas. J Therm Anal Calorim 110, 137–143 (2012). https://doi.org/10.1007/s10973-012-2245-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-012-2245-2