Abstract
A relatively unexplored goat’s milk fat and goat’s milk chocolate were investigated to enhance thermal properties of both. Differential scanning calorimetry, pressure differential scanning calorimetry and thermogravimetry were successfully used to determine polymorphic forms of goat’s milk fat, oxidative stability and the percentage composition of goat’s milk chocolate. The thermal profile and characteristic of the goat’s milk chocolate showed parameters very similar to dark chocolate. Significant differences were found in the induction times of goat milk fat and fat extracted from goat’s chocolate. For the goat’s milk fat, the value of activation energy (Ea) for a maximum temperature was almost identical to the Ea value obtained by the means of onset temperature. However significant difference was observed between values of Ea for the fat extracted from goat’s milk chocolate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Goat milk is an exquisite source of fatty acids (FA), protein and minerals [1]. The importance of goat milk as a functional food is related to its high digestibility and nutritional value, as well as its therapeutic and dietary characteristics [2]. An essential component that determines the better absorption of goat milk is small size fat globules whose composition varies significantly from other types of milk [3]. A high proportion of short- and medium-chain saturated fatty acids (SFA) such as butyric, caproic, caprylic and capric acid, and long-chain mono- and polyunsaturated fatty acids (MUFA and PUFA) provides better tolerance, especially among children and elderly people. The low percentage of allergies registered worldwide [4] makes goat milk very promising and valuable food ingredient, including chocolate. The presence of goat milk in chocolate allows to simultaneously implement essential nutrients into diet and avoid distinctive aroma and flavour characteristic for pure goat milk, not well accepted by consumers.
Regardless of the type of chocolate, its texture and appearance are key attributes in consumer choice [5]. Visual information characterizing objects, including gloss, colour, shape, roughness, surface texture, shininess and translucency, is summarized into the appearance attributes. The melting properties of chocolate are critical to their quality because they greatly influence consumer acceptability, appearance and storage stability of the product.
Chocolate, especially milk chocolate, is a very complex mixture of ingredients. Hence, it is difficult to analyse and relatively easy to falsify. Traditional analytical techniques used for analysing the amount of fat and protein are time-consuming and they require constant supervision. Therefore thermal analysis, such as differential scanning calorimetry (DSC) and thermogravimetry (TGA), is gaining more attention in the food analysis. DSC can be used in many ways, in either isothermal or non-isothermal mode, providing information about crystallization and oxidative stability, respectively. TGA is a simple, cost-effective and sensitive tool which can be used to determine the composition of dark and milk chocolate and consequently to control the quality of that product [6, 7].
During chocolate manufacture, the crystalline state and the proportion of solid fat present are important in determining the melting character in the final product [8]. Most of the fats can crystallize in different polymorphic forms which differ in stability due to distance and arrangement between fatty acid chains. Therefore, the melting profile can be a qualitative and quantitative picture of each chocolate as it reflects all varieties of fats and conditions applied during chocolate production. Melting profiles of chocolates are expected to differ due to various fatty acids composition and changeable amount of fine solid particles (sugar and cocoa) depending on the type of chocolate.
The aim of this work was to propose the thermogravimetry (TGA) and the derivative thermogravimetry (DTG) as fast and convenient techniques to determine the composition for goat milk chocolate, as well as differential scanning calorimetry (DSC), used to both, measure oxidative stability and characterize melting profile, for fats extracted from both, goat milk powder and goat milk chocolate.
Materials and methods
Samples
Goat milk powder and goat milk chocolate (65% cocoa liquor) were purchased in one of the well-known hypermarkets, at the healthy food section. The ingredients (according to the manufacturer’s declaration) found in chocolate are cocoa mass, raw cone sugar, goat milk powder, cocoa butter and salt. The average nutritional value per 100 g was sugar content—33%, fat content—46% (content of cocoa butter and cocoa mass was 64% minimum), protein—12% and salt—0.2%.
Fat extraction from goat milk powder and goat milk chocolate
Goat milk chocolate was ground separately before extraction. Fats were extracted according to the procedure described by Boselli et al. [9]. Approximately 30 g of the sample (either goat milk powder or ground goat milk chocolate) was homogenized with 100 mL of a chloroform/methanol solution (1/1 v/v) in a glass bottle with a screw-cap. The bottle was kept at 60 °C for 20 min before adding an additional 100 mL of chloroform. After 2 min of homogenization, the solution was filtered to get rid of undissolved pieces of chocolate. A filtrate was mixed thoroughly with 70 mL of 1 M KCl solution and left overnight at 4 °C in order to phase separation. The organic phase was collected, and the solvent was removed by the rotary evaporator at 40 °C. The cocoa butter, the goat milk fat and the fat isolated from goat milk chocolate were stored at − 18 °C until they were analysed.
DSC measurements for goat milk fat
The calorimetric measurements were performed with a Q200 DSC (TA Instruments, New Castle, DE, USA). Oxygen was used as the purge gas at a rate of 50 mL min−1. The instrument was calibrated in temperature and enthalpy with high-purity indium according to the procedure for standard DSC. A normal-pressure DSC cell was used. Fat samples of 3–4 mg were placed in open aluminium pans and inserted into the heating chamber of the DSC cell. The aluminium reference pan was left empty. Samples were heated with linear heating rates of 2.5, 4, 6, 7.5, 10, 12.5, 15 °C min−1. For each programmed heating rate (β, °C min−1), at least triplicate determinations were carried out. Each run was recorded on the instrument’s computer disc. When the run was completed, the onset oxidation temperature (Ton) was determined as the intersection of the extrapolated baseline and the tangent line (leading edge) of the recorded exotherm. The averages from measurements of Ton for each sample at a given temperature were determined as the intersection of the extrapolated baseline and the tangent line (leading edge) of the recorded exotherm. The maximum oxidation temperature (Tmax) was determined as the maximum exothermal peak of oxidation sample [10, 11].
Melting characteristics of goat milk chocolate, fats extracted from goat milk powder and goat milk chocolate
DSC measurements of melting characteristics were carried out with a Q200 DSC (TA Instruments, New Castle, DE, USA). For goat milk chocolate, approximately 3–4 mg of sample was placed in hermetically sealed aluminium pans with an empty pan as a reference. Samples were placed in the DSC immediately at a temperature set to 10 °C, under a nitrogen atmosphere. The temperature was then raised by 4 °C min−1 to 50 °C.
For isolated fats, approximately 3–4 mg were placed into aluminium pans with a lid and were hermetically sealed. An empty sealed aluminium pan was used as a reference, and the experiments were performed under a nitrogen flowing with a rate of 50 mL min−1 at normal pressure. Melted samples were heated to 80 °C and held for 10 min, in order to melt all the crystals and to erase the thermal memory. The samples were then cooled to − 80 °C at 10 °C min−1 and maintained at − 80 °C for 30 min. Then the melting (so-called second fusion) profiles were obtained by heating the samples to 80 °C at a heating rate of 10 °C min−1 [12, 13]. Peak temperature measurements were performed using the functions of the Universal Analysis Software (TA Instruments).
PDSC measurements for goat milk fat
Thermoanalytical measurements of oxidative stability of goat milk fat and fat extracted from goat milk chocolate were carried out using a DSC Q20 TA Instruments, coupled with a high-pressure cell. The sample (3–4 mg) was placed in aluminium pan, under an oxygen atmosphere, being pressurized in the isobaric model (1400 kPa). Experiments were performed at isothermal conditions (from 100, 110 and 120 °C). The oxidative induction time was obtained from PDSC curves. From resulting PDSC exotherms, the times to reach the peak maximum (τmax) were determined and used for the assessment of the oxidative stabilities of the samples [11].
Thermogravimetry analysis for goat milk fat
The thermogravimetric study was performed using a Discovery TGA (TA Instruments) analyser. The sample was placed in a platinum container. The measurements were made at temperature range 50–700 °C with a heating rate of 10 °C min−1, in both nitrogen and oxygen atmospheres, at a flow rate of 25 mL min−1. After obtaining TG curves showing temperature dependence on mass loss, the first derivate (DTG) was calculated [6, 14]. The method has been validated on real samples at three selected temperatures in triplicate. The experimental extended uncertainty reached a maximum 2%.
Statistical analysis
The data were reported as the mean ± standard deviation. One-way ANOVA was performed using the Statgraphics Plus, version 5.1 (Statistical Graphics Corporation, Warrenton, VA, USA). Differences were considered to be significant at a p value of 0.05, according to Tukey’s multiple range test. The experimental design was carried out with three replications.
Results and discussion
Melting characteristic by DSC
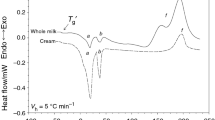
Goat’s milk chocolate is not so popular like milk or dark chocolates. During chocolate manufacture both, the crystalline state and the proportion of solid fat present, are important in determining the melting behaviour of final products. Differential scanning calorimetry (DSC) has been used to characterize changes in a chocolate melting profiles and measures the relative amounts of each crystalline state [8, 15]. The peaks corresponding to latent heat are observed in temperature ranges related to melting of specific polymorphs [16]. The most important ingredient of each chocolate is fat. The main kind of fat at all chocolates is constituted by cocoa fat. Melting profiles of goat fat and fat extracted from goat’s milk chocolate are presented in Fig. 1. Mild endothermic and wide peak in the range of temperature − 75.04–59.66 °C on melting curve of goat fat was observed. The percentage of MUFA’s is low in goat milk fat (24.46%) when compared with cow milk fat (27.73%); however, the PUFA level present in goat milk fat (4.67%) is higher than in cow milk fat (2.89%) and this may be due to species-specific differences [17, 18]. The first peak is caused by the low-melting point fraction of PUFA. The second, mild endothermic peak at a maximum temperature about 4.36 °C was noticed on the goat fat melting curve. According to Amara-Dali’s et al. [19] melting temperature corresponds to the melting of α polymorphic form of goat milk fat (melting range is − 7 to 5 °C). Amara-Dali’s et al. [20] investigated the crystallization of triacylglycerols (TG) in goat’s milk fat globules by using polarized light microscopy and the coupling of time-resolved synchrotron radiation X-ray diffraction (XRD) and high-sensitivity differential scanning calorimetry (DSC). The molecular organization of the solid fat phase was characterized for cooling rates between 3 and 0.1 °C min−1. Quenching of goat’s milk fat globules from 50 to either − 8 °C or 4 °C was also examined to identify the most unstable polymorphic forms of TG. Then, the melting behaviour of fat crystals was studied on the subsequent heating rate of 1 °C min−1. Triple chain length (3L: 68.6 − 70 Å) and double chain length (2L: 37 − 45.4 Å) structures were characterized and five polymorphic forms, α, sub-α, β′1, β′2 and β were identified. Polymorphic transitions were observed within goat’s milk fat globules as a function of time after quenching and as a function of temperature during heating. The maximum temperature of third most distinct (clearest) endothermic peak was observed at the temperature of 13.04 °C, and the last peak was characterized by maximum temperature at 26.38 °C (Fig. 1). The use of the synchrotron radiation and DSC revealed the existence of two different 3L (α and β′) and numerous 2L forms (α and β′) in anhydrous goat milk fat depending on crystallization conditions. Although the dominant stable form is β′, four different polymorphic subcell types, sub α, α, β′ and β, were observed [19]. The melting temperatures for changes polymorphic form subα, α and β′ occurred at temperature range: − 19 to − 7 °C; − 7 to 5 °C and 5 to 36 °C, respectively [19].
Sbihi et al. [21] investigated goat milk fat. They observed that during DSC analysis, melting curve exhibited two melting peaks at 15.4 °C and 38.7 °C, respectively. The large shoulder endothermic peak was attributed to the melting of triacylglycerol with a combination of unsaturated fatty acids, and short-chain fatty acids and medium-chain fatty acids, while the small shoulder endothermic peak is responsible for the melting of triacylglycerols with a combination of long-chain saturated fatty acids, mainly C16:0 and C18:0, and monounsaturated fatty acids with a trans configuration [22]. The transformation into the β′ polymorph form occurred rapidly when liquid phase was present during crystallization in the α polymorph mixture of high- and low-melting milk fractions [23].
Goat’s milk chocolate was characterized by the greater cocoa fat content than goat milk fat. That phenomena were noticeable in melting characteristic of fat extracted from goat’s milk chocolate (Fig. 1). Three endothermic peaks were observed on fat extracted from goat’s milk chocolate DSC melting curve. The first and the second peaks were characterized by very sharp course, the second peak being clearly sharper. The maximum temperature of the first and second endothermic peaks was observed at 17.78 and 20.06 °C, respectively. These temperatures indicated similar melting profile for I and II polymorphic forms of cocoa butter [5]. Cocoa butter has been characterized by five polymorphic forms: I (sub- or γ), II (α), III, IV (β′), V (β) and VI, the fifth, β, being the most stable. The maximum temperature of the third peak was noticed at 32.83 °C. The maximum temperature of the first and second peak occurred at higher value than for cocoa butter [24, 25]. The third peak could be the result of using goat milk fat in the production of chocolate.
The DSC curve of goat’s milk chocolate melting behaviour is shown in Fig. 2. The melting curve of goat’s milk chocolate is characterized by two endothermic peaks. The first peak, wide and mild, was observed at a maximum temperature of about 27.68 °C. The second peak was characterized by sharp and distinct course at a temperature about 34.05 °C. Goat milk contains about 4.1–4.3% lactose, 3.5–3.8% milk fat, 3.4–3.5% protein and 0.7–0.8% minerals [26, 27]. Milk fat triglycerides, dominated by saturated fatty acids, exhibit a different crystalline structure than adequate amounts of SFA represented by palmitic, stearic and oleic acid, found in the majority in cocoa butter [28]. Milk fat is mainly liquid at ambient temperature; hence, when used at up to 30% of the total fat content, it softens chocolate texture, slows setting and inhibits fat blooming effect [29]. Milk fat is prone to oxidation and influences shelf life [25, 28]. According to Afoakwa et al. [30], melting the most desirable polymorphic form V should be carried out at a temperature range of 32–34 °C. The maximum melting temperature of goat’s milk chocolate is very similar to the temperature of melting V form of cocoa butter. Hussain et al. [31] researched the best blend of cocoa butter substitute and genuine cocoa butter to provide milk chocolate of a similar characteristic to pure milk chocolate. The maximum melting temperature of milk chocolate occurred at a level of about 29.9 °C, while a goat’s milk chocolate was characterized by a higher melting temperature. However, based on the previous scientific reports, it can be expected that the addition of sugars [30] and a well-conducted tempering process can both influence the melting characteristic of goat’s milk chocolate.
Thermogravimetry
The thermogravimetric and first derivative data of the goat milk fat, fat extracted from goat’s milk chocolates and goat’s milk chocolate, under the nitrogen and oxygen atmosphere, are presented in Figs. 3, 4 and 5.
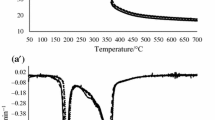
The TG and DTG diagrams of goat milk fat in nitrogen and oxygen are presented in Fig. 3a, b. The TG curve in nitrogen was characterized by only one event, corresponding to 98.95% mass loss (Fig. 3a), while on DTG curves one distinct peak at temperature range 285.16–475.32 °C was observed. The shape and course of goat milk fat TG and DTG curves in nitrogen are very similar to cow milk fat TG and DTG curves [31], even though the goat milk fat is characterized by five polymorphic forms [19]. In Fig. 3b, TG and DTG diagrams of goat milk fat in oxygen are presented. In contrast to a nitrogen atmosphere, a TG diagram performed under oxygen atmosphere was characterized by four transitions; therefore, a DTG curve showed four stages of goat milk fat decomposition at maximum temperature peaks: 278.13; 409.77; 417.99; and 502.12 °C. Sbihi et al. [21] investigated goat milk fat by using TGA/DTG in a dry air atmosphere. The characteristic of the TGA/DTG diagrams showed three stages of decomposition for goat milk fat, with a maximum temperature at 316.5, 440 and at 541.3 °C. Our results obtained in pure oxygen are comparable because of the third peak on Fig. 3b which is visibly a part of the second peak. According to Szabo et al. [32], the first stage of the thermal decay is the decomposition of the unsaturated fatty acids, mainly C18:1, C18:2 and C18:3 and the SCFA (C4–C6). This stage is considered the most important and represents the initial phase of triacylglycerol degradation. In this phase, the oxidation of PUFAs occurs. The next stages of the curve represent the decompositions of trans isomers of fatty acids and saturated fatty acids. The TGA/DTG curves obtained from fat extracted from goat’s milk chocolate in nitrogen and oxygen atmosphere are presented in Fig. 4a, b. The TG diagram is characterized by one transformation in nitrogen. The maximum temperature observed on a DTG curve is 412.15 °C which is higher than for goat milk fat. The fat extracted from goat’s milk chocolate is a mixture of cocoa butter and goat milk fat. Ostrowska-Ligęza et al. [33] obtained TGA/DTG diagrams for cocoa butter in nitrogen with the maximum temperature at 414 °C. The blend of two fats caused a slight decrease in maximum peak temperature, yet similar value may suggest a bigger contribution of cocoa butter than goat milk fat in the fat phase. The TGA/DTG curves representing a fat extracted from goat’s milk chocolate under oxygen atmosphere are shown in Fig. 4b. Similarly to Fig. 3b, four stages of transition were obtained characterized by maximum temperatures 304.48 °C, 400.92 °C, 413.74 °C and 500.34 °C. The fourth peak can be classified as mild, while the other three peaks were characterized by a very sharp and distinct course. Yet, differences between shape and course of TGA/DTG curves for goat milk fat and fat extracted from goat’s chocolate in oxygen atmosphere were significant enough. The TGA/DTG curves for goat’s milk chocolate in nitrogen and an oxygen atmosphere are presented in Fig. 5a, b. On TGA diagram in nitrogen, two events were observed; however, on DTG curve three steps were distinguished. In the case of the first peaks on DTG curve, a temperature ranged from about 175.19 to 247.98 °C. The maximum temperature for this peak occurred at 205.56 °C. Materazzi et al. [6] had studied TGA curves for dark chocolates and their ingredients in both, nitrogen and air atmospheres. According to Materazzi et al. [6], the first step of the DTG curve was obtained by sugar transformation. The similar results, provided by Ostrowska-Ligęza et al. [33], were determined by TGA/DTG analysis of dark and milk chocolates at different processing stages. According to the manufacturer’s declaration, the sugar content was 33%, the fat content was 46% and content of cocoa butter and cocoa mass was minimum 64%. The goat milk fat came only from 12% addition of goat milk powder. The content of sugar in goat’s milk chocolate, calculated on the basis of the DTG diagrams, was lower than in milk chocolates [32]. According to Materazzi et al. [6] and Ostrowska-Ligęza et al. [33], the second step is the release of the cocoa liquor and the final is related to the mass loss of cocoa butter. The second step in goat’s milk chocolate was very distinct and the range of temperature 259.79 to 340.60 °C was very wide, like in dark chocolates. The third peak, observed at a temperature ranged from 345.51 to 411.06 °C, was characterized by distinct course and sharp shape (Fig. 5b). The content of fat in goat’s milk chocolate was comparable to the level of fat content in dark chocolates (on the basis of the DTG curves for these products) [32]. According to Materazzi [6], the nitrogen purging flow gives a clear qualitative profile yet the quantitative interpretation of the analysis is not allowed since inert flows depress the complete decomposition, without a final constant mass value. A decomposition is obtained in goat’s milk chocolates by changing an atmosphere of measurements to oxygen. The shape of the TG curve showed three stages for goat’s milk chocolate. The first transition was very mild, the second was sharper course and the third was characterized by a very distinct shape finishing with a plateau at 331.49 °C (Fig. 5b). The mass loss of goat’s milk chocolate finished very rapidly. Similar results for dark chocolate were obtained by Ostrowska et al. [33]. The thermal decomposition of sugar was observed on the DTG curve in the temperature range from 184.67 to 230.49 °C. The second transition temperature on DTG curve ranged from 243.73 to 313.42 °C, corresponding to the thermal decomposition of cocoa liquor (Fig. 5b). The presence of the last peak indicated that the fat (cocoa butter and goat milk fat blend) was oxidized. The DTG curve in oxygen for goat’s milk chocolate showed intense peak at temperature range from 310.31 to 326.99 °C, characterizing fat in goat’s milk chocolate. Cocoa butter and goat milk fat formed the eutectic mixture in goat’s milk chocolate; however, the goat milk fat contribution was visibly smaller than cocoa butter amount. The thermal behaviour of goat’s milk chocolate was very similar to dark chocolate. However, information on the thermal properties of this chocolate is scarce.
Kinetics and induction time of oxidation
The study of thermostability and fat thermo-oxidation is an important problem of theoretical and practical importance. The experimental onset and maximum temperature (Ton and Tmax) obtained at seven heating rates (2.5; 4; 6; 7.5; 10; 12.5 and 15 °C min−1) are shown in Table 1. The Ton and Tmax experimental values as a function of heating rates (β) were recalculated on onset and maximum temperatures (Ton and Tmax). Putting values of Ton or Tmax into the Eq. (1):
where a and b are adjustable coefficients [10, 12, 34], a linear correlation was found (R2 > 0.99 in each case). The regression analyses of the data are listed in Table 2. The kinetic parameters of the oxidation process were calculated based on the assumption that the amount of heat released in the heat mode at a given time is proportional to the number of reagents present. For established initiation conditions, fat oxidation reactions occurring in excess of oxygen were first-order reactions [10]. If the maximum temperature increases linearly like the heat coefficient, then the Ozawa–Flynn–Wall method [10, 11, 35,36,37] can be used to determine the activation energy Ea and the pre-exponential factor Z. By using the transformation of constant temperatures (Tmax maximum, Ton onset), determined using the DSC method, the approximate value of the activation energy is calculated (2):
where R is the gas constant, β the heating rate (°C min−1) and T the temperature (K).
The value of the pre-exponential factor Z from the Arrhenius equation was calculated from the dependence (3):
The kinetic parameters for the goat milk fat and fat extracted from goat’s milk chocolate are presented in Table 2.
The goat milk fat onset temperatures were represented by lower values than temperatures of fat extracted from goat’s chocolate. The same tendency for maximum temperatures was observed (Table 1). The activation energy Ea for a goat milk fat was at the level of 73.17 (Ton) and 72.26 (Tmax) kJ mol−1, that is almost identical (Table 2). Significant differences were observed between the values of Ea for the fat extracted from goat’s milk chocolate. Chocolate fat is a mixture of goat milk fat and cocoa butter. Cocoa butter is characterized by bigger stability than goat milk fat. Saldaña and Martínez-Monteagudo [38] determined different kinds of fats and oils using differential scanning calorimetry. The activation energy for cocoa butter at isothermal conditions (160 °C, oxygen atmosphere) is equal to 120.5 kJ mol−1. Sbihi et al. [21] investigated goat milk fat. The fatty acids composition influenced on thermal stability of goat milk fat. The authors stated that goat milk fat contained a lower percentage of saturated fatty acid (67.15%). Hence, compared to cow milk fat, goat milk fat has higher digestibility. This is related to the lower mean milk fat globule size [39] and the higher content of short- and medium-chain fatty acids [40, 41]. A characteristic feature of the cocoa butter is a high content of saturated fatty acids—palmitic (C16:0), stearic (C18:0) and monounsaturated oleic (C18:1) [42]. Kowalska et al. [43] obtained the values of 26.2%, 34.4%, 37.3% and 2.1% for palmitic acid, stearic acid, oleic acid and linoleic acid (C18:2), respectively. The content of saturated fatty acids has an influence on the thermal stability of fats. Induction time at 100, 110 and 120 °C values obtained for goat milk fat and fat extracted from goat’s chocolate are presented in Table 2. The results show that the goat milk fat is characterized by a lower induction time than fat extracted from goat’s chocolate. The differences between the values of induction time were significant. Induction time for goat milk fat at 120 °C was equal to 21.95 min which was about three times lower than for fat extracted from goat’s chocolate—62.89 min. A similar pattern for all induction temperatures was observed. The cocoa butter in mixture caused the increase in induction time. The induction time for cocoa butter, investigated at 120 °C by Rancimat method, gave results from 9 to 15 h [44]. Cocoa butter was characterized by six polymorphic forms and goat milk fat by five polymorphic forms. Polymorphism had an influence on the thermal stability of fats.
Conclusions
The melting characteristic determined for fat extracted from goat’s milk chocolate proved a higher content of cocoa butter in the mixture than a content of goat milk fat. On the DSC melting curves of goat milk fat and fat extracted from goat’s milk chocolate, the differences were observed. The course of melting diagram of goat’s milk chocolate differed from milk chocolate. TGA and DTG curves for goat milk fat and fat extracted from goat’s milk chocolate indicated differences between these fats. TGA and DTG diagrams for goat’s milk chocolate in nitrogen and in oxygen allowed to conclude about the amount of chocolate ingredients. The TGA and DTG curves for goat milk fat in oxygen indicated the existence of high-melting triacylglycerols. Although the goat milk fat is characterized by a high content of unsaturated fatty acids, the initial temperatures of the oxidation process (onset) were relatively high. Therefore, this fat can be characterized by high oxidative stability. DTG diagrams for goat’s milk chocolate showed a low content of sugar. The quality of goat’s milk chocolate is satisfactory. This kind of chocolate can be recommended for consumption due to its hypoallergenic properties, good taste and slightly better digestibility in comparison with typical milk chocolate.
References
Lad SS, Aparnathi KD, Bhavbhuti MM, Velpula S. Goat milk in human nutrition and health: a review. Int J Cur Micr Appl Sci. 2017;6(5):1781–92.
Fonseca CR, Bordin K, Fernandes AM, Rodrigues CE, Corassin CH, Cruz AG, Oliveira CA. Storage of refrigerated raw goat milk affecting the quality of whole milk powder. J Dairy Sci. 2013;96:4716–24.
Gantner V, Mijić P, Baban M, Škrtić Z, Turalija A. The overall and fat composition of milk of various species. Mljekarstvo. 2015;65(4):223–31.
Albenzio M, Campanozzi A, D’Apolito M, Santillo A, Pettoello Mantovani M, Sevi A. Differences in protein fraction from goat and cow milk and their role on cytokine production in children with cow’s milk protein allergy. Small Rumin Res. 2012;105:202–5.
Beckett ST. The science of chocolate. 2nd ed. Cambridge: The Royal Society of Chemistry; 2008.
Materazzi S, De Angelis Curtis S, Vecchio Ciprioti S, Risoluti R, Finamore J. Thermogravimetric characterization of dark chocolate. J Therm Anal Calorim. 2014;116:93–8.
Pardauil J, de Molfetta F, Braga M, de Souza L, Filho G, Zamian J, da Costa C. Characterization, thermal properties and phase transitions of amazonian vegetable oils. J Therm Anal Calorim. 2017;127:1221–9.
Afoakwa E, Paterson A, Fowler M, Vieira J. Characterization of melting properties in dark chocolates from varying particle size distribution and composition using differential scanning calorimetry. Food Res Int. 2008;41:751–7.
Boselli E, Velazco V, Caboni MF, Lercker G. Pressurized liquid extraction of lipids for the determination of oxysterols in egg-containing food. J Chromatogr A. 2001;917:239–44.
Ostrowska-Ligęza E, Wirkowska M, Kowalski B. Thermokinetic analisys of corn grain fat by differential scanning calorimetry. Zywn-Nauk Technol Ja. 2009;1(62):128–39 (in Polish).
Wirkowska-Wojdyła M, Bryś J, Górska A, Ostrowska-Ligęza E. Effect of enzymatic interesterification on physiochemical and thermal properties of fat used in cookies. LWT-Food Sci Technol. 2016;74:99–105.
Wirkowska M, Ostrowska-Ligęza E, Górska A, Koczon P. Thermal properties of fats extracted from powdered baby formulas. J Therm Anal Calorim. 2012;110:137–43.
Marudova M, Momchilova M, Antova G, Petkova Z, Yordanov D, Zsivanovits G. Investigation of fatty acid thermal transitions and stability in poultry pates enriched with vegetable components. J Therm Anal Calorim. 2018;133:539–47.
Teixeira G, Ávila S, Meira Silveira J, Ribani M, Hoffmann Ribani R. Chemical, thermal and rheological properties and stability of sapucaia (Lecythis pisonis) nut oils. A potential source of vegetable oil in industry. J Therm Anal Calorim. 2018;131:2105–21.
Walter P, Cornillon P. Lipid migration in two-phase chocolate systems investigated by NMR and DSC. Food Res Int. 2002;35:761–7.
Instrumentation McFarlane I. Instrumentation. In: Beckett ST, editor. Industrial chocolate manufacture and use. New York: Chapman & Hall; 1999. p. 347–76.
Ander BP, Dupasquier CM, Prociuk MA, Pierce GN. Polyunsaturated fatty acids and their effects on cardiovascular disease. Exp Clin Cardiol. 2003;8:164–72.
Kalyan S, Meena S, Kapila S, Sowmya K, Kumar R. Evaluation of goat milk fat and goat milk casein fraction for anti-hypercholesterolaemic and antioxidative properties in hypercholesterolaemic rats. Int Dairy J. 2018;84:23–7.
Amara-Dali WB, Lesieur P, Artzner F, Karray N, Attia H, Ollivon M. Anhydrous goat’s milk fat: thermal and structural behaviors studied by coupled differential scanning calorimetry and X-ray diffraction. 2. Influence of cooling rate. J Agric Food Chem. 2007;55(12):4741–51.
Amara-Dali WB, Lopez C, Lesieur P, Ollivon M. Crystallization properties and polymorphism of triacylglycerols in goat’s milk fat globules. J Agric Food Chem. 2008;56(12):4511–22.
Sbihi HM, Nehdi IA, Tan CP, Al-Resayes SI. Characteristics and fatty acid composition of milk fat from Saudi Aradi goat. Grasas Aceites. 2015;66(4):101–8.
Ten Grotenhuis E, Van Aken G, Van Malssen K, Schenk H. Polymorphism of milk fat studied by differential scanning calorimetry and real-time X-ray powder diffraction. J Am Oil Chem Soc. 1999;76(9):1031–9.
Cisneros A, Mazzanti G, Campos R, Marangoni A. Polymorphic transformations in mixtures of high- and low-melting fractions of milk fat. J Agric Food Chem. 2006;54:6030–3.
Merken G, Vaeck S. Étude du polymorphisme du beurre de cacao par calorimetrie DSC. Lebensm Wiss Technol. 1980;13(6):314–7.
Afoakwa E, Paterson A, Fowler M. Factors influencing rheological and textural qualities in chocolate a review. Trends Food Sci Tech. 2007;18:290–8.
Park Y. Goat Milk: composition, characteristics. In: Pond WG, Bell N, editors. Encyclopedia of animal science. New York: CRC Press; 2010. p. 1280–9.
Rasheed S, Mabood Qazi I, Ahmed I, Durrani Y, Azmat Z. Comparative study of cottage cheese prepared from various sources of milk. Proc Pak Acad Sci B Life Environ Sci. 2016;53(4):269–82.
Haylock SJ, Dodds TM. Ingredients from milk. In: Beckett ST, editor. Industrial chocolate manufacture and use. Oxford: Blackwell Science; 1999. p. 137–52.
German JB, Dillard CJ. Fractionated milkfat. Food Technol. 1998;52:33–7.
Afoakwa E, Paterson A, Fowler M, Vieira J. Microstructure and mechanical properties related to particle size distribution and composition in dark chocolate. Int J Food Sci Tech. 2009;44:111–9.
Hussain N, Agus BAP, Rahim SNFA, Halim HAS. Comparison of quality characteristics between compound and pure milk chocolate. MOJ Food Process Technol. 2018;6(3):292–6.
Szabo M, Chambre D, Iditoiu C. TG/DTG/DTA for the oxidation behaviour characterization of vegetable and animal fats. J Therm Anal Calorim. 2012;110:281–5.
Ostrowska-Ligęza E, Górska A, Wirkowska-Wojdyła M, Bryś J, Dolatowska-Żebrowska K, Shamilowa M, Ratusz K. Thermogravimetric characterization of dark and milk chocolates at different processing stages. J Therm Anal Calorim. 2018;134:623–31.
Górska A, Ostrowska-Ligęza E, Szulc K, Wirkowska M. A differential scanning calorimetric study of β-lactoglobulin and vitamin D3 complexes. J Therm Anal Calorim. 2012;110:473–7.
Flynn JH, Wall LA. A quick direct method for determination of activation energy from thermogravimetric data. J Polym Sci B Polym Lett. 1966;4:323–8.
Ozawa T. Kinetic analysis of derivative curves in thermal analysis. J Therm Anal. 1970;2:301–24.
Baokun Q, Zhang Q, Sui X, Wang Z, Li Y, Jiang L. Differential scanning calorimetry study—assessing the influence of composition of vegetable oils on oxidation. Food Chem. 2016;194:601–7.
Saldaña MD, Martínez-Monteagudo SI. Oxidative stability of fats and oils measured by differential scanning calorimetry for food and industrial applications. In: Elkordy AA, editor. Applications of calorimetry in a wide context. differential scanning calorimetry, isothermal titration calorimetry and microcalorimetry. London: Sunderland University Published; 2013. p. 445–74.
Tatar V, Mootse H, Sats A, Mahla T, Kaart T, Poikalainen V. Evaluation of size distribution of fat globules and fat and protein content in Estonian goat milk. Agron Res. 2015;13(4):1112–9.
Ceballos LS, Morales ER, de la Torre Adarve G, Castro JD, Martínez LP, Remedios Sampelayo MR. Composition of goat and cow milk produced under similar conditions and analysed by identical methodology. J Food Compos Anal. 2009;22(4):322–9.
Yurchenko S, Sats A, Tatar V, Kaart T, Mootse H, Jõudu I. Fatty acid profile of milk from Saanen and Swedish Landrace goats. Food Chem. 2018;254:326–32.
Sa’eed B. Pro-health properties of chocolate—the food of the gods. Bezp Hig Żywn. 2005;10:34–5 (in Polish).
Kowalska J, Bzducha A, Derewiaka D, Kopańska K, Nitek A. Evaluation of the authenticity of selected chocolates. Zywn-Nauk Technol Ja. 2008;4(59):74–9 (in Polish).
Application Bulletin 204/2 e. Oxidation stability of oils and fats – Rancimat method.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Dolatowska-Żebrowska, K., Ostrowska-Ligęza, E., Wirkowska-Wojdyła, M. et al. Characterization of thermal properties of goat milk fat and goat milk chocolate by using DSC, PDSC and TGA methods. J Therm Anal Calorim 138, 2769–2779 (2019). https://doi.org/10.1007/s10973-019-08181-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08181-0