Abstract
Simultaneous thermoanalytical techniques were used for the characterization of the thermal decomposition of ketoprofen—active substance and tablets. DTA and DSC curves showed that ketoprofen melts before the decomposition. A kinetic study regarding the ketoprofen—active substance’s thermal decomposition was performed under non-isothermal conditions and in a nitrogen atmosphere at five heating rates: 2.5, 5, 7.5, 10 and 15 °C min−1. The kinetic parameters of thermal decomposition process were obtained from TG/DTG curves using the following differential methods: Friedman isoconversional, Chang, respectively, integral methods: Flynn–Wall–Ozawa, Kissinger–Akahira–Sunose, Coats–Redfern and Madhusudanan. The careful treatment of the kinetic parameters obtained in certain thermal conditions was confirmed to be necessary as well as a different strategy of experimental data processing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
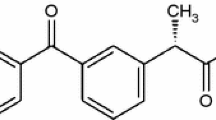
Ketoprofen 2-(3-benzoylphenyl)-propionic acid, with the formula:

is a non-steroidal anti-inflammatory drug (NSAID), therapeutically used in inflammatory and painful diseases of rheumatic and non-rheumatic origin.
The anti-inflammatory activity of NSAIDs and most of its other pharmacological effects are related to the inhibition of the conversion of arachidonic acid to prostaglandins, which are mediators of the inflammatory process. Ketoprofen is a potent inhibitor of cyclo-oxygenase (COX-1 and COX-2) in vitro and in vivo, thereby decreasing the synthesis of prostaglandins, prostacyclin and thromboxane products. Inhibition of the COX-2 system results in anti-inflammatory action, whilst inhibition of the COX-1 enzyme system results in anti-inflammatory action as well as gastric irritation [1–5].
The thermal analysis is a routine method applied for drugs characterization and it is useful in the preformulation stage in the development of solid dosage forms. Research in thermal decomposition of drugs is of great interest in developing new products since it is often necessary to predict degradation rates at marketing temperatures from collected data on accelerated processes studied at elevated temperatures.
Thermal analytical techniques, such as thermogravimetry/derivative thermogravimetry (TG/DTG) and differential scanning calorimetry (DSC) techniques, provide important information regarding the physical properties of the pharmaceutical compounds (purity, polymorphism, thermal stability, compatibility, kinetic analysis, etc.) [6–11].
Thermogravimetry is an analytical, quantitative and comparative method, capable of producing fast and reproducible results. It can be used in the quality control of drugs, with a view to the improvement of the final product and for the determination of drug quality via technological parameters [12–15].
Differential scanning calorimetry, which is frequently used instead of the DTA, can be used in pharmaceutical research as an analytical tool of great importance for the identification and purity testing of active drugs and especially to elucidate the miscibility/incompatibility with its effects on thermal stability, yielding results rapidly and efficiently [7, 16–19].
The thermal stability is a very important factor since the determination of the temperature range in which a certain medicine substance (drug) is stable regarding its structure, as well as its pharmaceutical action is crucial for stocking drugs, for its technological transformations and for the obtaining technology of the right formulas.
Thermal behaviour of the substances can also be characterized by the kinetic analysis. One main purpose of the kinetic analysis of solid decomposition is to determine reaction mechanism(s) and to calculate Arrhenius parameters. There are two ways to do this, using either isothermal or non-isothermal kinetic analysis, each category of methods having advantages and disadvantages in the determination of kinetic parameters [20–25].
In our previous articles we provided the importance and utility of the kinetic analysis in estimation on the thermal behaviour of different pharmaceuticals [26–30].
Thermogravimetry, during which the change of mass of a sample heated at constant rate is recorded and plotted versus temperature, is an effective method of studying thermal stability and for determining the kinetic parameters of the decomposition of drugs and medicines [31–33].
In this article, thermoanalytical techniques were used to study thermal behaviour of ketoprofen, active substance and tablets, respectively, kinetic analysis of the active substance under non-isothermal (dynamic) conditions.
Experimental
Materials
The substances examined by thermal analysis were:
-
ketoprofen—active substance (AS)
-
ketoprofen—tablets (M) or drug.
The active substance was obtained from Terapia S.A./Ranbaxy, Cluj-Napoca, Roumania as pure compound, able to be used for medical purposes. The pharmaceuticals (tablets) were commercial products, containing different excipients like: starch, colloidal silicon dioxide, talc, citrate of triethyl, microcrystalline cellulose, magnesium stearate, povidone, lactose monohydrate, etc.
Methods
TG/DTG/DTA experiments were performed with a Netzsch-STA 449 TG/DTA instrument in the temperature range 20–500 °C, using platinum crucibles with ≈20 mg of samples under dynamic nitrogen atmosphere (20 mL min−1) and the heating rates of 2.5, 5, 7.5, 10 and 15 °C min−1.
Differential scanning calorimetry curves were recorded with a Netzsch differential scanning calorimeter, model DSC-204, using aluminium crucibles with samples of 3 mg, under dynamic nitrogen atmosphere (50 mL min−1) and a heating rate of 10 °C·min−1, up to a temperature of 500 °C.
Results and discussions
Thermal behaviour of ketoprofen
Some of the thermal curves of the two substances, obtained under dynamic temperature conditions at heating rate of 10 °C min−1 and a nitrogen atmosphere, are presented in Figs. 1, 2 and 3.
The DTA curve of active substance (Fig. 1) shows an endothermic sharp event between 89.4 and 128.6 °C indicating the melting (T peak = 96.8 °C), which corresponds to the values indicated in the literature (94–97 °C). In this temperature range the TG/DTG curves did not show mass loss.
The second broad event, observed in DTA curve was also an endothermic one which corresponds to the thermal decomposition process. This is confirmed by TG/DTG curves that indicate thermal decomposition in the following temperature range and mass loss: 235–400 °C (Δm = 86%). For the decomposition process the thermal curves indicate the following temperatures of the peaks: DTGpeak = 361.4 °C; DTApeak = 365 °C. The DTA data combined with TG allow evidencing a thermal stability of ketoprofen active substance up to 235 °C.
Also, the DSC curve (Fig. 2) confirms the purity of ketoprofen AS by a sharp endothermic peak at 96.7 °C that corresponds to melting followed by thermal decomposition.
The Fig. 3 shows the TG/DTG and DTA curves of the commercial pharmaceutical product using the same experimental conditions in relation to active substance with a different thermal profile caused by the presence of excipients and the possible interaction of them.
The DTA curve shows two endothermic events (T peaks = 94.8 and 145.6 °C), which are due to melting, respectively, to lactose dehydration. The third endothermic event, T peak = 209.5 °C, corresponds to the melting of lactose which stabilizes the ketoprofen.
TG/DTG curves indicate that thermal decomposition of the ketoprofen tablets takes place in different temperature ranges causing the respective mass losses: Δm 1 = 2.6% (DTGpeak = 55.2 °C) and Δm 2 = 1.7% (DTGpeak = 144.1 °C) corresponding to excipient dehydration: microcrystalline cellulose [34, 35], magnesium stearate [36, 37] and lactose monohydrate [38, 39], Δm 3 = 9.1% (DTGpeak = 224.2 °C) and Δm 4 = 62.6% characterized by a broad endothermic peak (DTGpeak = 289.5 °C) with two right shoulders (DTGpeaks = 322.1 and 367.1 °C).
The melting of lactose is followed by the decomposition of this (DTGpeak = 224.2 °C) and the main process of the decomposition takes place in the temperature range 257.8–475 °C. This is a complex process because the decomposition of ketoprofen tablets is accompanied by the decomposition of some excipients as starch and magnesium stearate.
Over 400 °C, respectively, 475 °C, the TG/DTG curves indicate a slow and continuous mass loss caused by elementary carbon formation from the previous steps, as a consequence of the rupture of the aromatic ring.
Kinetic analysis
Also, the thermal stability was characterized by using the kinetic parameters, on the basis of the kinetic study performed under non-isothermal conditions, which sustained the present facts.
The kinetic parameters, the rate constant (k), the activation energy (E), the pre-exponential factor (A) and reaction order (n) were determined from the TG/DTG curves, by using the differential methods, Friedman isoconversional (Fd) [23] and Chang [30], respectively, integral methods, Flynn–Wall–Ozawa (FWO) [40], Kissinger–Akahira–Sunrose (KAS) [24], Coats–Redfern (CR) [24] and Madhusudanan (Md) [41].
From the equation of reaction rate:
for non-isothermal conditions dα/dt is replaced with βdα/dT, where β is the heating rate, giving:
The isoconversional Friedman method is based on the equation:
In order to evaluate the activation energy more precisely, the term ln(dα/dT) was obtained by numerical derivation of the curve α vs. T with respect to T and subsequent taking logarithms.
For α = constant and using various heating rates, the plot ln(βdα/dT) vs. (1/T) is linear (Fig. 4). The values of the activation energy as obtained from the slopes of the straight lines are listed in Table 1.
The general equation of the reaction rate for non-isothermal conditions at constant heating rate is generally written as:
where g(α) is the conversion integral and p(x) is the temperature integral.
From the general equation, Eq. 4, using Doyle’s approximation [24]:
the equation corresponding to the Flynn–Wall–Ozawa’s isoconversional method was obtained:
This method is based on the measurement of the adequate temperature to certain values of the conversion α, for experiments effectuated to different rates of heating β.
The plot ln β vs. (1/T) is linear (Fig. 5). From the slopes of the straight lines (−E/R), the values of the activation energy (E) were obtained (Table 1).
The Kissinger–Akahira–Sunose method, sometimes called the generalized Kissinger method, is one of the best isoconversional methods and it is based on the equation:
This method utilizes the adequate temperatures (T α) to certain values of the conversion α for experiments effectuated to different rates of heating, β.
From the slopes of the straight lines obtained by the graphic representation of the \( \ln \left( {{\beta \mathord{\left/ {\vphantom {\beta {T_{\alpha }^{2} }}} \right. \kern-\nulldelimiterspace} {T_{\alpha }^{2} }}} \right) \) vs. (1/T α), the activation energy was determined. The values of kinetic parameters determined by means of the integral methods are presented in Table 1.
The values obtained by the three methods are in good agreement and the weak variation of E vs. α indicates a single reaction mechanism for the decomposition processes.
From the Eq. 1, by logarithmation it is obtained the equation corresponding to the Chang’s method:
where α is the conversion degree, t is the time, f(α) is the reaction model and T is the temperature.
The different differential functions f(α) were substituted in Eq. 8 and the relations were linearized, therefore, obtaining three-parametric linear equations: z = a + bx + cy.
The use of TableCurve3D Software (http://www.sigmaplot.com/products/tablecurve3d/tablecurve3d.php) can determine the kinetic triplet (E, ln A, n) through the estimation of the three parameters: a, b, c.
For different f(α), the most probable to describe the kinetic model, it was calculated the regression coefficient. Based on this values and also because of the activation energy’s sign (the activation energy must have physical sense), it was chosen the function: f(α) = (1 − α)n, which represents the reaction order model, and n = 1/2 (Table 2). From the graphic representation on the experimental data (Fig. 6.), the values of E, A and n were determined (Table 2).
As shown in Table 1, for 0.1 ≤ α ≤ 0.9, the activation energy’s values fluctuate around the average values of: (82.5 ± 3.6), (81.1 ± 2.6) and, respectively, (74.6 ± 1.5) kJ mol−1, and they are in fair good agreement with the value obtained with the Chang method (Table 2).
For the kinetic model, f(α) = (1 − α)n, mentioned to the Chang method, and frequently met in the decomposition of the drugs, the method Coats–Redfern uses the following equations:
The graphic representation of the left side versus 1/T leads to a straight line for n = 1/2 (Fig. 7) and from the slopes and the intercepts of the straight lines, the values of E and A were obtained. The rate constant (k) was calculated by the Arrhenius equation, the temperature utilized was the temperature which corresponds to the maximum of the decomposition (T β2.5 = 588 K, T β5 = 607 K, T β7.5 = 620 K, T β10 = 631 K and T β15 = 637 K). The values of the kinetic parameters are presented in Table 3.
A similar method to Coats–Redfern one is the Madhusudanan method which uses the following equations:
The values of E, A and k were determined in the same mode as for the Coats–Redfern method and these values are presented in Table 3.
For ketoprofen—active substance, the values of kinetic parameters determined by means of the integral methods are in fair good agreement between them, respectively, with the values determined by means of the differential methods.
As been mentioned, thermoanalytical curves corresponding to the ketoprofen tablets indicate the existence of a complex decomposition process because of the presence and of the excipients’ possible interactions. This leads to the impossibility of assignment of the values to the kinetic parameters which range in a broad interval (E = 77–224 kJ mol−1; ln A = 15–31 min−1) of a particular process; because of this, the assignment would be inaccurate and lack scientific support.
Conclusions
There was performed a thermal study (thermal behaviour and kinetic determinations) under non-isothermal conditions for the ketoprofen—active substance and their pharmaceutical form.
There have been observed differences between the thermal curves of the pure compound and those of the pharmaceutical product, due to the presence of the excipients in drug and due to the possible interactions with the active substance.
The study of thermal behaviour of the two ketoprofen samples evidenced that the presence of excipients increases the thermal stability of this drug. It is shown by rather higher decomposition temperatures and larger decomposition ranges. This behaviour is confirmed through the kinetic study that indicated lower values of activation energy for the standard sample (active substance). By the values of kinetic parameters, the kinetic study shows a weak thermal stability of ketoprofen—active substance.
The values of the kinetic parameters, determined with differential and integral methods, are in fair good agreement, and they are situated in a narrow range (E = 73–82 kJ mol−1; ln A = 13.5–14.4 min−1) and this fact indicates the correctness of the applied methods.
The concordance of the values for the kinetic parameters determined and the mode of dependence of the activation energy as against the conversion degree show the fact that the thermal decomposition process of the ketoprofen active substance is indeed an unitary process, but relatively complex in the case of ketoprofen tablets.
The kinetic study through values of kinetic parameters (especially E) can be used in the preformulation and production steps for quality control of medicines, together with the melting point which characterizes their purity.
References
Dutta S, Padhye S, McKee V. Structural characterization and SOD activity of copper-oxaprozinate. Inorg Chem Commun. 2004;7:1071–4.
Fini A, Fasio G, Benetti L, Ghedini V. Thermal analysis of some diclofenac salts with alkyl and alkylhydroxy amines. Thermochim Acta. 2007;464:65–74.
Barbato F, Cappello B, La Rotonda MI, Miro A, Quaglia F. Diclofenac/β-cyclodextrin binary systems: a study in solution and in the solid state. J Incl Phenom Macrocycl Chem. 2003;46:179–85.
Ying YC, Yi L, Cheng ZJ, Dan Z. Inhibitory effect of copper complex of indomethacin on bacteria studied by microcalorimetry. Biol Trace Elem Res. 2008;122:82–8.
Kafarska K, Czakis-Sulikowska D, Wolf WM. Novel Co(II) and Cd(II) complexes with non-steroidal anti-inflammatory drugs. Synthesis, properties and thermal investigation. J Therm Anal Calorim. 2009;96:617–21.
Bernardi LS, Oliveira PR, Murakami FS, Silva MAS, Borgmann SHM, Cardoso SG. Characterization of venlafoxine with pharmaceutical excipients. J Therm Anal Calorim. 2009;97:729–33.
Moyano MA, Broussalis AM, Segall AI. Thermal analysis of lipoic acid and evaluation of the compatibility with excipients. J Therm Anal Calorim. 2010. doi: 10.1007/s10973-009-0351-6.
Moura EA, Correia LP, Pinto MF, Procopio JVV, de Sousa FS, Macedo RO. Thermal characterization of the solid state and raw material fluconazole by thermal analysis and pyrolysis coupled to GC/MS. J Therm Anal Calorim. 2010. doi: 10.1007/s10973-009-0473-X.
Oliveira PR, Bernardi LS, Murakami FS, Mendes C, Silva MAS. Thermal characterization and compatibility studies of norfloxacin for development of extended release tablets. J Therm Anal Calorim. 2009;97:741–5.
Bannach G, Cervini P, Cavalheiro ETG, Ionashiro M. Using thermal and spectroscopic data to investigate the thermal behaviour of drugs and excipients by unique calculations. J Therm Anal Calorim. 2010. doi: 10.007/s10973-009-0595-1.
Avula SG, Alexander K, Riga A. Predicting eutectic behavior of drugs and excipients by unique calculations. J Therm Anal Calorim. 2010;99:655–8.
Salvio Neto H, Barros PAF, de Souza Carvalho MF, Matos RJ. Thermal analysis of prednicarbate and characterization of thermal decomposition product. J Therm Anal Calorim. 2010;102:277–83.
Felix SF, da Silva CCL, Angnes L, Matos RJ. Thermal behaviour study and decomposition kinetics of salbutamol under isothermal and non-isothermal conditions. J Therm Anal Calorim. 2009;95:877–80.
Macedo OR, Aragao SFC, do Nascimento GT, Macedo CMA. Application of thermogravimetry in the quality control of chloramphenicol tablets. J Therm Anal Calorim. 1999;56:1323–7.
Macedo OR, de Souza GA, Macedo CMA. Application of thermogravimetry in the quality control of mebendazole. J Therm Anal Calorim. 1997;49:937–41.
Nunes SR, Semaan SF, Riga TA, Cavalheiro GTE. Thermal behavior of verapamil hydrochloride and its association with excipients. J Therm Anal Calorim. 2009;97:349–53.
Freire DF, Aragao SFC, de Lima AFT, Raffin NFM. Compatibility study between chlorpropamide and excipients in their physical mixtures. J Therm Anal Calorim. 2009;97:355–7.
Mura P, Gratteri P, Faucci TM. Compatibility studies of multicomponent tablet formulations. DSC and experimental mixture design. J Therm Anal Calorim. 2002;68:541–51.
Oliveira GGG, Ferraz GH, Matos RSJ. Thermoanalytical study of glibenclamide and excipients. J Therm Anal Calorim. 2005;79:267–70.
Ortega A. A simple and precise linear integral method for isoconversional data. Thermochim Acta. 2008;474:81–6.
Chrissafis K. Kinetics of thermal degradation of polymers. Complementary use of isoconversional and model-fitting methods. J Therm Anal Calorim. 2009;95:273–83.
Saha B, Maiti AK, Ghoshal AK. Model-free method for isothermal and non-isothermal decomposition kinetics analysis of PET sample. Thermochim Acta. 2006;444:46–52.
Dickinson CF, Heal GR. A review of the ICTAC kinetics project, 2000: Part 1. Isothermal results. Thermochim Acta. 2009;494:1–14.
Dickinson CF, Heal GR. A review of the ICTAC kinetics project, 2000: Part 2. Non-isothermal results. Thermochim Acta. 2009;494:15–25.
Budrugeac P. Differential non-linear isoconversional procedure for evaluating the activation energy of non-isothermal reactions. J Therm Anal Calorim. 2002;68:131–9.
Tiţa B, Marian E, Tiţa D, Vlase G, Doca N, Vlase T. Comparative kinetic study of decomposition of some diazepine derivatives under isothermal and non-isothermal conditions. J Therm Anal Calorim. 2008;94:447–52.
Tiţa B, Fuliaş A, Marian E, Tiţa D. Thermal behaviour of acetylsalicylic acid—active substance and tablets. Kinetic study under non-isothermal conditions. Rev Chim (Bucureşti). 2009;60:419–23.
Tiţa B, Fuliaş A, Marian E, Tiţa D. Thermal stability and decomposition kinetics under non-isothermal conditions of sodium diclofenac. Rev Chim (Bucureşti). 2009;60:524–8.
Fuliaş A, Tiţa B, Bandur G, Tiţa D. Thermal decomposition of some benzodiazepines under non-isothermal conditions. Kinetic study. Rev Chim (Bucureşti). 2009;60:1079–83.
Tiţa B, Fuliaş A, Rusu G, Tiţa D. Thermal behaviour of indomethacin—active substance and tablets kinetic study under non-isothermal conditions. Rev Chim (Bucureşti). 2009;60:1210–5.
Li X, Wu Y, Gu D, Gan F. Thermal decomposition kinetics of nickel (II) and cobalt (II) azo barbituric acid complex. Thermochim Acta. 2009;493:85–9.
Giron D, Goldbronn C. Use of DSC and TG for identification and quantification of the dosage form. J Therm Anal Calorim. 1997;48:473–83.
Howell BA. Utility of kinetic analysis in the determination of reaction mechanism. J Therm Anal Calorim. 2006;85:165–7.
Tomassetti M, Catalani A, Rossi V, Vecchio S. Thermal analysis study of the interactions between acetaminophen and excipients in solid dosage forms and in some binary mixtures. J Pharm Biomed Anal. 2005;37:949–55.
Marini A, Berbenni V, Pegoretti M, Bruni G, Cofrancesco P, Sinistri C. Drug-excipient compatibility studies by physico-chemical techniques; The case of Atenolol. J Therm Anal Calorim. 2003;73:547–61.
Corvi Mora P, Cirri M, Mura P. Differential scanning calorimetry as a screening technique in compatibility studies of DHEA extended release formulations. J Pharm Biomed Anal. 2006;42:3–10.
Barboza F, Vecchia DD, Tagliari MP, Silva MAS, Stulzer HK. Differential scanning calorimetry as a screening technique in compatibility studies of acyclovir extended release formulations. Pharm Chem J. 2009;43:363–8.
Desai R, Shaikh MM, Dharwadkar SR. Preformulation compatibility studies of etamsylate and fluconazole drugs with lactose by DSC. J Therm Anal Calorim. 2003;71:651–8.
Balestrieri F, Magrì AD, Magrì AL, Marini D, Sacchini A. Application of differential scanning calorimetry to the study of drug-excipient compatibility. Thermochim Acta. 1996;285:337–45.
Genieva SD, Vlaev LT, Atanassov AN. Study of the thermooxidative degradation kinetics of poly (tetrafluoroethene) using iso-conversional calculation procedure. J Therm Anal Calorim. 2010;99:551–61.
Madhusudanan PM, Krishnan K, Ninan KN. New equations for kinetic analysis of non-isothermal reactions. Thermochim Acta. 1993;221:13–21.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tiţa, D., Fuliaş, A. & Tiţa, B. Thermal stability of ketoprofen—active substance and tablets. J Therm Anal Calorim 105, 501–508 (2011). https://doi.org/10.1007/s10973-010-1187-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-1187-9