Abstract
The concept of weighted mean activation energy has been used to assess the reactivity of Thar coal in terms of pyrolytic and combustion behavior using non-isothermal thermogravimetry. The samples were characterized as low sulfur and high volatile lignite to subbituminous coal. Modified Coats–Redfern method was applied to analyze the kinetic data of both processes. Thermal degradation of the samples studied was explained by three independent first-order kinetic steps. The good correlation coefficients for the independent first-order reaction models show the complexity of the overall process. The activation energy for pyrolysis reaction ranges from 19.20–63.55 to 23.68–54.49 kJ mol−1 for combustion profile. The samples of different rank were classified applying chemometric approach on the reactivity parameters. Principal component analysis was found as a statistical tool to organize the coal samples in accordance to ASTM classification. This study broadens the scope of thermal analysis in the field of coal utilization by assessing coal reactivity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coal reactivity is considered as one of the major significant aspects in understanding coal gasification and other conversion processes such as carbonization, combustion, and liquefaction [1]. Coal undergoes a series of physical and chemical changes when heated in an inert or oxidized environment. Physical changes include softening, resolidification, surface morphology, porosity of the solid particle whereas chemical changes involve bond breaking and recombination [2, 3]. During coal combustion, pyrolysis takes place first and coal turns into semi-coke and volatiles including gas and oil. Pyrolysis is important for coal beneficiation along with the characterization of the generated char. It also affects on ignition behavior and flame stability. Combustion characteristics are essential for coal utilization especially in power plants and in the designing of industrial coal-fired boiler furnaces [4]. Although pyrolysis and combustion of coal has been studied worldwide [5–7] but extensive research is still the subject of prime importance especially for the newly explored reserves either valuable as fuel in natural form or whose products may have to be considered beneficial as a substitute for oil or natural gas.
Pakistan has emerged as one of the leading country of the world after the discovery of huge reserves of coal at Tharparkar district of Sindh province. The estimated reserves of coal (175.5 billion tonnes) suggests it an important resource for supporting economical development, especially in the situation of severe shortage of electricity with the limited resource of natural gas in the country and heavily rely on imported oil. Unfortunately the huge reserves of Thar are not exploited yet although it was discovered in 1989. Now the serious efforts are underway at the Government level for its proper utilization. Classical mining is the most common and popular technology for the extraction of coal but it is not feasible economically at Thar; as three water aquifers were found at an average depth of 50, 120, and more than 200 m [8]. Experts suggested underground gasification of coal (UCG) for electricity generation as the best solution for the utilization of unmineable coal at Thar. In UCG, the coal seam is dried, ignited, and finally pyrolyzed. Understanding the kinetics of coal reactivity is crucial to predict process performance of UCG and other industrial processes of coal utilization. Coal reactivity is usually measured by thermogravimetric analysis (TG) using isothermal and non-isothermal methods. There are certain limitations of both methods. Isothermal TG is considered more appropriate than non-isothermal TG for the thermal decomposition of general chemical reactions as the activation energy value changes with heating rate [9, 10]. Thermal degradation of coal is a complex process, and a number of consecutive parallel reactions are involved in the process. Hence, it is difficult to analyze the kinetic parameters in a shorter period of time using isothermal TG. However, non-isothermal TG method with certain modification is recommended for kinetic measurement of coal reactivity by several authors, as it is sufficiently simple and accurate, studying the reaction in one single experimental run, and achieves complete char conversion in a shorter period of time [11, 12].
In this study, pyrolysis and combustion decomposition of Thar coal is performed with the aim to investigate overall reactivity of the samples using kinetic approach for non-isothermal thermogravimetry. At the moment, low-rank coals are classified according to gross calorific value (GCV) on moist, mineral-matter-free (mmf) basis, and high-rank coals according to fixed carbon content on mmf basis (standard classification of coals, ASTM D 388-99). The classification of coal requires sophistication in coal testing methods concerning to its utilization beyond the analytical techniques commonly available to fuel technologists. One of such sophisticated test is the assessment of coal reactivity. Cumming [13] has suggested that the most significant parameters of coal reactivity profile are volatile matter, activation energy, and peak temperature (at which the sample is losing weight at the maximum rate). The important outcome of this study is the use of chemometric tool to enhance the scope of thermal analysis by classifying the samples of different rank. For this purpose, the significant parameters of coal reactivity are exploited to constitute homogeneous clusters of the same rank.
Experimental
Twenty-five coal samples from Thar were collected in replicates from different depths (120–240 m) and boreholes of Block V of Thar coalfield. The samples were air dried (ASTM D-3302), grinded, and pulverized to 60 mesh (ASTM D-2013). Proximate analysis, Total sulfur content, and GCV were measured using TG analyzer (TGA 2000A, Las Navas, Spain), Sulfur determinator (SC-32, LECO), and adiabatic bomb calorimeter (Parr 6300, USA) in accordance with ASTM D-5142, D-4239, and D-5865, respectively. The reproducibility in the results was ensured by analyzing the samples in replicates. The percentages of carbon, hydrogen, and nitrogen were calculated using empirical formula derived by Carpenter and Diederichs [14]. The percentage of oxygen was calculated by difference. The experimental data of as-determined (ad) basis was converted to as-received (ar) basis, dry, ash-free (daf) basis (ASTM D-3180-89) and moist, mineral-matter-free (mmf) basis (ASTM D-388-99).
The pyrolysis studies have been conducted at the samples dried at 105 °C. The samples were pyrolyzed under 30 mL min−1 N2 flow and heating rate of 10 °C min−1 from 110 to 800 °C. For combustion studies, the samples were oxidized by 20 mL min−1 O2 flow and heating rate of 16 °C min−1 from 110 to 1000 °C. To strengthen the utility of this study, the combustion and pyrolysis data of the samples of different rank were selected from the literature [15–19]. Principal component analysis (PCA) was applied on pyrolysis and combustion data of indigenous and foreign coal samples to identify the patterns. The similarities and differences in coal quality on the basis of reactivity parameters (activation energy, volatile matter, and peak temperature) were also highlighted by classifying the samples accordingly. PCA is a well-known statistical technique used previously by several workers to interpret the properties of coal by removing the dimensional redundancy from data without losing the integrity of information [20–22]. For this purpose, chemometric measurements were performed using statistical software Minitab version 14.
Results and discussion
Classification and characterization of coal
The samples are classified in accordance with GCV on the moist, mineral-matter-free basis (ASTM D388-99). The proximate and ultimate compositions of the six representative samples are shown in Table 1. Sample 1–3 and 5 are ranked as lignite while sample 4 and 6 are ranked as subbituminous. The quality of coal is assessed on as-received basis according to coal quality rank index [23]. All samples have high volatile matter (≥ 29%) and low calorific values (−10800 btu/lb). Sample 2 and 3 are characterized as high ash (16+%); the remaining samples have low ash (−10%). Sulfur content of samples 1 is high (1.5%+), 2 and 6 are medium (0.8–1.5%), and 3, 4, and 5 are low (−0.8%). The ultimate analysis of Thar coal shows total carbon 58.00–73.74%, hydrogen 6.52–11.18%, nitrogen 1.62–3.88%, and oxygen 16.67–25.16% on dry-ash-free (daf) basis.
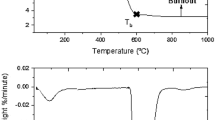
Non-isothermal TG pyrolysis was carried out in an inert atmosphere after the removal of inherent moisture. Figure 1 represents TG and DTG curves and their characteristic temperatures. The beginning temperature of coal pyrolysis (T i) was considered as the temperature at which 5% volatiles has been lost; (T p) was taken as the temperature of maximum rate of weight loss and the end temperature (T f) was measured when 85% of the volatiles has been emitted [24]. The pyrolysis study of 25 coal samples shows that T i of the coal samples studied varied from 315 to 450 °C. On the other hand, T p and T f are varied in the temperature range of 545–700 and 750–950 °C, respectively. The volatilization occurs due to the breaking of chemical bonds. The difference in T p values may be attributed to the inherent composition of coal; as discussed in earlier investigations that the reactivity in terms of organic matter conversion is dependent on the nature of coal [24, 25].
Kinetics of pyrolysis and combustion reactions
It is evident from Fig. 1 (inset) that the plot of rate versus weight of unburned coal is linear showing pyrolysis as first-order reaction. It may simply be expressed as
where A is the pre-exponential factor, E a represents activation energy, and R is the universal gas constant. x is the extent of pyrolysis conversion or weight loss fraction at time t, it is calculated as:
where w i is the initial mass of coal sample before pyrolysis; w t is the mass at time t, and w f is the final mass remained at the end of pyrolysis. The measurement of reactivity of coal via pyrolysis is a function of two main components; i.e., heating rate and volatiles fraction emitted. Haoquan and coworkers [25] used Coats–Redfern method derived by integration of Eq. 1 for a constant heating rate β [26]
For a single first-order reaction model (SFOR), the plot of left side of Eq. 3 versus \( \frac{1}{T} \) should result in a straight line of slope equals \( \, - \frac{{E_{\text{a}} }}{R} \). The equation was applied on all samples and it was found that all plots were characterized by three regions of linearity. It indicates that three independent first-order reactions are involved at different temperature regions (Fig. 2 shows the curve for sample 2 as the representative). Hence, it is concluded that the pyrolysis of Thar coal cannot be described by SFOR model. To resolve the complexity of the pyrolysis, the method of weighted mean activation energy (E m) was adopted, and activation energy of the complex process was calculated as described by Cumming [13]. He has focused on the contribution of each region to the overall reactivity of coal in addition to the sum of apparent activation energy of each independent step. He has proposed a simple relation for the calculation of multistep pyrolysis reactions:
where F 1 to F n are the weight fractions of the combustible content of the samples pyrolyzed during each region of linearity; and E 1 to E n are the individual apparent activation energies of each independent step. The factor \( \ln \,\left[ {\frac{{ - \ln \left( {1 - x} \right)}}{{T^{2} }}} \right] \) in Eq. 3 was recalculated separately for each stage. The typical results for sample 2 with good correlation coefficient values are shown in Fig. 3. The values of E a were calculated from the slopes of each line. E 1, E 2, and E 3 were attributed to the different reaction mechanisms involved as the temperature was increased. Activation energy values for the combustion reaction of six selected samples were calculated using the same treatment in an oxidized environment. Tables 2 and 3 show the activation energy values for pyrolysis and combustion processes, respectively. The difference in activation energy values is attributed to the mineral matter contents of the samples [18]. The mean activation energy for pyrolysis and combustion were calculated as 35.50 and 34.27 kJ mol−1, respectively. The values are in good agreement with the E m values of low quality coal [3, 13, 15, 16, 18]. Low activation energy of Thar coal samples shows that the reaction needs less energy from the surroundings. The repeatability of thermal profile curves were found to be good with peak temperature varying by not more than 5 °C for replicate measurements.
Chemometric analysis
PCA was used as a statistical tool to classify the coal samples of different ranks on the basis of activation energy, volatile matter, and peak temperature (Tables 2 and 3). PCA has been performed in several steps. The first step comprises of the selection of number of significant components. Kaiser criterion with eigen value ≥1 was selected as the numbers of significant principal components [27]. According to this criterion, the reduced dimensionability of the descriptor space is two because the subsequent eigen values were less than 1.
For pyrolysis reaction, eigenanalysis of the Correlation matrix showed 62.20% of the total variability for first principal component (PC) which comprises of activation energy, volatile matter, and peak temperature. The second PC (explaining 21.60% of the total variance) is mainly made up of volatile matter and activation energy. The third component (16.2% of the total variance) is correlated with activation energy and peak temperature (Table 4). The cumulative contribution of first and second PC in pyrolysis data is 83.8%. Figure 4 shows score plot of the two most important PCs.
For combustion behavior, eigenanalysis of correlation matrix explained 76.10% of the total variability for first PC which is made up of activation energy, peak temperature, and volatile matter. The second PC (explaining 17.40% of the total variance) is made up of activation energy and peak temperature. The third component (6.5% of the total variance) is correlated with volatile matter and peak temperature. Since, the first-two PC represents 93.5% cumulative variance; therefore, Fig. 5 represents the score plot of the representative components. The PCA shows remarkable groupings of samples with respect to their ranks based on their thermal reactivity parameters. It is important to note that peak temperature, previously used as the indicator of coal reactivity, gives only a rough idea about the reactivity and becomes very uncertain in the case of multiple-peak profiles as commonly observed for low-rank coals. Tables 2 and 3 show reactivity parameters of Thar coal along with the data reported for coals of different regions of the world. All samples were classified by ASTM standard classification by selecting fixed carbon and calorific value as the fingerprint criterion for high- and low-rank samples, respectively. The significance of PCA is that it provides an alternate method to ASTM classification and classifies the samples accordingly on the basis of reactivity parameters. It was found to be a simple visual representation of the complex data. Therefore, it is suggested that PCA is a more concise parameter to discriminate different ranks of coal on the basis of their reactivity profile because it covers all the major features of thermal degradation of coal.
Conclusions
Thar coal samples of block V are classified as lignite to subbituminous rank. Kinetic studies of devolatilization and oxidation of the samples show that thermal decomposition of Thar coal is a complex heterogeneous process and involves a number of parallel and consecutive reactions. The mean values of activation energy for pyrolysis and combustion reactions are 35.50 and 34.27 kJ mol−1, respectively. Low activation energy values categorize it as a reactive coal. PCA was found as a good chemometric tool to classify the samples in accordance with ASTM standard classification of coal.
References
Cakal GO, Yucel H, Guruz AG. Physical and chemical properties of selected Turkish lignites and their pyrolysis and gasification rates determined by thermogravimetric analysis. J Anal Appl Pyrolysis. 2007;80:262–8.
Tromp PJJ, Kapteijn F, Moulijn JA. Characterization of coal pyrolysis by means of differential scanning calorimetry. 2. Quantitative heat effects in a H2 and in a CO2 atmosphere. Fuel Process Technol. 1989;23:63–74.
Elbeyli IY, Piskin S. Combustion and pyrolysis characteristics of Tuncbilek lignite. J Therm Anal Calorim. 2006;83:721–6.
Azhagurajan A, Nagaraj P. An experimental analysis of coal aluminium mixture in coal fired furnace. J Therm Anal Calorim. 2009;98(1):253–9.
Mohalik NK, Panigrahi DC, Singh VK. Application of thermal analysis techniques to assess proneness of coal to spontaneous heating. J Therm Anal Calorim. 2009;98:507–19.
Fangxian L, Shizong L, Youzhi C. Thermal analysis study of the effect of coal-burning additives on the combustion of coals. J Therm Anal Calorim. 2009;95(2):633–8.
Ozbas KE. Effect of particle size on pyrolysis characteristics of Elbistan lignite. J Therm Anal Calorim. 2008;93(2):641–9.
http://www.sindhmines.gov.pk/pdf/TharCoalResources-brochure.pdf. Accessed 14 Sep 2010.
Brown ME, Maciejewski M, Vyazovkin S, Nomen R, Sempere J, Burnham A, Opfermann J, Strey R, Anderson HL, Kemmler A, Keuleers R, Janssens J, Desseyn HO, Li C-R, Tang TB, Roduit B, Malek J, Mitsuhashi T. Computational aspects of kinetic analysis. Part A: The ICTAC kinetics project-data, methods and results. Thermochim Acta. 2000;355:125–43.
Vyazovkin S, Wight CA. Model-free and model-ftting approaches to kinetic analysis of isothermal and nonisothermal data. Thermochim Acta. 1999;340–341:53–68.
Otero M, Gomez X, Garcia AI, Moran A. Non-isothermal thermogravimetric analysis of the combustion of two different carbonaceous materials coal and sewage sludge. J Therm Anal Calorim. 2008;93(2):619–26.
Russell NV, Beeley TJ, Man CK, Gibbins JR, Williamson J. Development of TG measurements of intrinsic char combustion reactivity for industrial and research purposes. Fuel Process Technol. 1998;57:113–30.
Cumming JW. Reactivity assessment of coals via a weighted mean activation energy. Fuel. 1984;63:1436–40.
Carpenter RC, Diederichs H. Experimental engineering. 8th ed. Wiley: New York; 1913. p. 507.
Ceylan K, Karaca H, Onal Y. Thermogravimetric analysis of pretreated Turkish lignites. Fuel. 1999;78:1109–16.
Guldogan Y, Bozdemir TO, Durusoy T. Effect of heating rate on pyrolysis kinetics. Energy Sour. 2000;22:305–12.
Elbeyli IY, Piskin S. Pyrolysis kinetics of Turkish bituminous coals by thermal analysis. Turk J Eng Environ Sci. 2004;28:233–9.
Kok MV. Temperature-controlled combustion and kinetics of different rank coal samples. J Therm Anal Calorim. 2005;79:175–80.
Kizgut S, Cuhadaroglu D, Toroglu I. Thermogravimetric characterization of Turkish bituminous coals for combustion. Turk J Chem. 2003;27:521–8.
Tesch S, Otto M. Application of principal-component analysis to the interpretation of brown coal properties. Fuel. 1995;74(7):978–82.
Figueiredo N, Coutinho CA. Analysis of principal components of coal ash by X-ray fluorescence spectrometry. Metal. ABM. 1979;35(260):453–7.
Khare P, Baruah BP. Chemometric analysis of trace elements distribution in raw and thermally treated high sulphur coals. Fuel Process Technol. 2010;91(11):1691–701.
Hurst MA, The International Coal Encyclopedia; Coal services Ltd., Time off set Pte Ltd. 1st ed. of the international encyclopedia (ICE). vol. 1, 1990. p 11.
Cigdem S, Sadriye K. Effect of mineral matter on the burning profile of lignites. Thermochim Acta. 1996;285:35–46.
Quanrun L, Haoquan H, Qiangu Z, Shengwei Z, Guohua C. Effect of inorganic matter on reactivity and kinetics of coal pyrolysis. Fuel. 2004;83:713–8.
Coats AW, Redfern JP. Kinetics parameters from thermogravimetric data. Nature. 1964;201:68–9.
Kaiser HF. The application of electronic computer to factor analysis. Educ Psychol Meas. 1960;20:141–51.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarwar, A., Nasiruddin Khan, M. & Azhar, K.F. Kinetic studies of pyrolysis and combustion of Thar coal by thermogravimetry and chemometric data analysis. J Therm Anal Calorim 109, 97–103 (2012). https://doi.org/10.1007/s10973-011-1725-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1725-0