Abstract
Coal is an important fuel used in boiler furnaces. There are problems like unburnt coal and solid wastes like ash contain arsenic, selenium, chromium and cadmium while using it. In order to avoid all such difficulties, aluminium metal powder in various grain sizes mixed with pulverized coal and burned. aluminium metal powder is one of the pyrotechnics having higher calorific value and low ignition temperature. The thermal behavior of aluminium powder along with coal is recorded in DTA. The collected ashes were tested in Scanning Electron Microscope and X-Ray Diffraction meter. The SEM results show that coal ash is having granular and regular structure. All the particles have a size range from 5 to 8 μm. On the other hand, the aluminium coal mixture ash shows a fibrous matrix and the particles are irregular. In XRD graph, the peaks in the graph show orientation of atoms in particular plane and angle. The coal ash has a lot of peaks, but the maximum count value reaches only to 340.97. However the value of counts reaches a maximum of 1,539.06 for aluminium ash. This denotes high atom orientation in a single lattice plane.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of Boiler is inevitable nowadays. Almost all boilers have coal fired furnace. The coal is used in the pulverized form. The calorific value and the composition of coal vary from each allotment. This gives a lot of problem during operation of furnace.
The main defect in this system is that considerable portion of coal is unburnt. The efficiency of operation also depends upon the ash content of the coal.
To sustain combustion in the furnace and also to reduce the unburnt mass and incomplete combustion of coal (Production of carbon monoxide), attempt is made by using some other fuel of high calorific value in addition to coal. Aluminium powder is selected because of its high calorific value (30,000 kJ/kg) and low ignition temperature (as low as 65 °C).
While using aluminium powder along with coal, the unburnt mass can be minimized. The use of oil in the intermediate stage can be completely eliminated, thereby increase in productivity.
The mass of oil and coal used can thus be minimized and hence savings in the cost. Moreover, production of CO is eliminated as complete combustion prevails.
The usage of main fuel coal is minimized and so, the emission of CO2 and SO2 and solid wastes like heavy metals arsenic, selenium, chromium and cadmium, carcinogenic organic compounds and radioactive elements is also reduced.
The main objective of this research work is as follows:
Use of aluminium powder along with coal is to maintain sustained combustion. Therefore use of oil in the intermediate stages due to failure of feeding of coal is to be eliminated.
Complete combustion of coal reduces the mass of unburnt coal and this gives substantial savings and also maintains pollution free atmosphere by reducing CO.
Ultimately it is aimed for
-
i.
Reduction of usage of coal
-
ii.
Savings of foreign exchange
-
iii.
Environmental friendly
-
iv.
Productivity increase
Problems in use of coal
The largest source of mercury pollution is coal fired power plants. Solid wastes from coal fired power plant also contain heavy metals like arsenic, selenium, chromium and cadmium, carcinogenic organic compounds and radioactive elements. The 600 plus coal fired power plants in the United States, releases 98,000 pounds (44 metric tonnes) of mercury into the air each year [1].
Although pollution scrubbers in modern smoke stacks reduces air pollution, they do nothing to help the coal miners who die each year in mine accidents or from diseases brought on by breathing hazardous coal dust [2].
Several European countries have begun to lead the transition away from coal. In Germany, coal use has been cut in half since 1990. Coal use in the United Kingdom has dropped by 46% over the same period [2].
In the coal fired furnace, ignition energy needed to ignite the coal is high. Almost it consumes 30% of the calorific value of the fuel. The disturbance in coal feeding also disturbs the temperature state in the furnace and leads to reduced productivity.
The release of sulphur dioxide during the burning of coal is an unwanted hazard. Therefore minimizing the amount of coal used not only is eco friendly and saves in cost.
The best alternative fuel found as aluminium metal powder.
Aluminium is being used as a fuel in fireworks, explosives due to high combustion temperature (730.98 kJ/g) [3, 4]. It is one of the propellants which is used in rocket fuels [11]. Its ignition temperature is 65 °C only [4].
Therefore, aluminium is selected for this study. Unfortunately, the literature in such mixture of fuel is very few.
Aluminium powder as a substitute
DSC studies on the effect of aluminium particle size led to multiple exothermic activity. Onset temperature increased with increase in particle size and remain as constant. The decrease in the aluminium particle size increases the heat of reaction [2].
As combustion temperature of aluminium metal powder is very high in the order of 3,500 °C, it is used as one of the fireworks fuels and propellants in rocket [4, 5]. During burning, it emits high heat energies (32,32,210 J), enhances the explosive properties. Impulse of explosion, improves with the addition of aluminium metal powder [6–8]. Exhaustive studies on blast parameters and properties of explosive compositions by varying the percentage of ingredients, particularly aluminium powder, in high energy formulations have been carried out [9].
The large amount of energy liberated by subsequent reactions of aluminium with primary detonation products, however, maintains a high pressure for longer period [10]. The blast effect is improved by adding aluminium powder to the explosive compositions [11].
Aluminized” Liquid Hydrocarbon Propellant Fuels [11] and Aluminized Gel Propellants are used in the liquid and gas propellants. It is observed that the burning life of single aluminum particle in oxygen is inversely proportional to the square of the diameter and nano aluminum powder has burning rates twice that of micron size powders.
Aluminium has electronegativity of 1.5. This is lower than for the coal (2.5). This special property refers the capability of receiving electrons and thus oxidized. Stochiometric ratio for aluminium is 16.40 with air or 3.8 with oxygen [11].
Valencies of elements normally encountered in combustion phenomena. The element valency for aluminum is 3. Values of equivalence ratio confirm the expected feature regarding fuel richness [12].
Materials and methods
Availability of aluminium metal powder
The aluminium metal powder of various grades is readily available in Sivakasi, Tamilnadu, India, because it is one of the main raw materials for the fireworks. The manufacturers of this aluminium metal powder are catering nearly 700 fireworks and various other companies in and around Sivakasi.
Preparation of different particle sizes of aluminium powder
The aluminium powder was sieved using different meshes of numbers 150 (118 µ), 200 (63 µ), 300 (40 µ) and 400 (35 µ) into five different particle sizes such as −100 + 150, −150 + 200, −200 + 300, −300 + 400 and −400.
Preparation of different mixtures of coal/Al powder
The five different particle sizes of aluminium powder referred above were mixed thoroughly in three different ratios: 90:10, 80:20, 70:30 of coal and Al powder respectively. Also, pyrotechnic aluminium powder which is used in fireworks (999 grade which comprises particles below 40 µ size) was also mixed in the following ratios: 90:10:0 and 70:15:15 of coal, pyrotechnic powder and −400 mesh atomized powder respectively.
The method of mixing is as follows:
The powders are not mixed as per ASTM standard [D2396-94 (2004)]. But, the homogeneous mixing was obtained using the mechanical aided mixture machine. The sieves used for this mixing are satisfying BSS standard. The number of mixtures required was calculated with help of Design of Experiment software. The use of aluminium powder is limited to 30% in the mixture, because of the safety aspects in the use of powder.
All the ratios are calculated by percentage of weight. The details are shown in the Table 1.
Thermal analysis
Bomb calorimeter
The mixtures are tested in a bomb calorimeter to find its heat of combustion. The results are tabulated in the Table 2.
Thermal analysis in DTA
Experiments were conducted at non-isothermal conditions with heating rate of 10 °C/min under atmospheric air up to 1,000 °C.
Details of Thermogravimetric/Differential thermal analyser
-
Make: Perklin Elmer
-
Model: Pyris Diamond
-
Temperature range
-
Standard system: Room temperature to 1,100 °C
-
High temp system: Room temperature to 1,500 °C
-
Weight measurement: Horizontal differential balance method
-
Sample weight: Max 200 mg
-
Heating range: 0.01–200.0 °C/min
-
Before starting the experiments, the temperature calibration was done.
Experimental conditions:
The following parameters are kept constant during the experiments:
-
Heating rate: 10 °C/min
-
Gas flow rate: 2 ml/min
-
Gas: Air
The temperature calibration before conducting experiments was performed following manufacturer’s instructions.
Being the initial study to check the possibility of inclusion of aluminium powder in coal, only three samples are selected by the stratified sampling basis. Since the results are conclusive, future study may include testing of all samples under consideration.
The graph between the heating temperature and the temperature difference is given for coal with mixture of aluminium powder (−150 + 200) in the ratio of 80/20 and 90/10 is given in the following Figs. 1, 2 and 3. The burn out and peak temperatures are shown in the Table 3.
Results of DTA
DTA behavior of clear coal sample is analysed and given in Fig. 1. It has an initial onset point at around 250 °C. After that, the output rises steeply to a peak and reaches a maximum at 527 °C. After that, the temperature difference decreases and at 657 °C, the reaction reaches a steady state.
For sample 13, which is an 80/20 mixture, the onset point is at 224 °C. This is 26 °C lesser than that for coal. This emphasizes the fact that Al powder addition has made the mixture to ignite more quickly. The peak is reached at the same temperature as that of coal, at 527 °C. However, before reaching the final point, aluminium undergoes melting since its melting point, 660 °C, is reached. This allows a dip of output temperature from 634 to 647 °C.
Similarly the sample 14 also has the onset point reached at 227 °C. This is around 23 °C lesser than that for coal. The peak is reached at 524 °C almost the same as that of coal and sample 13. The melting of aluminium takes place from 632 to 647 °C, similar to that of sample 13. However the amount of decrease in temperature of sample during melting is decreasing with decrease in aluminium composition. This melting process is major disadvantage of the usage of aluminium powder.
Ash test
Percentage of ash found in the samples are given in Table 4.
The results like energy released, amount of CO2 produced, the weight of ash formed and thermal behavior of aluminium powder along with coal with respect to particle size of aluminium powder were very promising. This emphasizes the fact that Al powder addition has made to ignite the mixture more quickly.
Particle morphology of ash in SEM
The study under scanning electron microscope (SEM) gives morphological analysis which provides information about the physical relationships of the size, crystallinity and juxtaposition of the phases present.
Two SEM images of coal, at 1000× and 3000×, and three SEM images of sample 13, at 1000×, 3000× and 5000× were obtained.
Specifications:
-
Make: HITACHI Model - S 3000H.
-
Maximum magnification: 300,000×
-
Resolution: 3.5 nm
-
Image display: Secondary electron image
-
Automatic functions: Auto brightness and contrast, auto focus and stigmatism, auto gun alignment, auto start
-
Samples: Solid samples (pellets, powders, thin films, metallic samples)
Different structures of coal ash and aluminium mixture ash are shown in Figs. 4, 5, 6, 7 and 8
The SEM results show that for coal, the ash is granular with regular structure. All the particles have a size range from 5 to 8 μm. On the other hand, the aluminium mixture sample shows a fibrous matrix. The particles are irregular and of different particle sizes.
These inferences from the images lead us to incline towards the fact that aluminium ash has some irregular and needle shaped structures which may be dangerous to the boiler parts due to abrasive effect of ash while being removed.
Crystal structure of ash analysis in XRD
X-ray crystallography is a technique in crystallography in which the pattern produced by the diffraction of X-rays through the closely spaced lattice of atoms in a crystal is recorded and then analyzed to reveal the nature of that lattice. This generally leads to an understanding of the material and molecular structure of a substance.
The pattern of powder diffraction peaks can be used to quickly identify materials (from the JCPDS pattern database), and changes in peak width or position can be used to determine crystal size, purity, and texture.
The XRD has a sample spinner in which the sample is placed.
The X-ray beam is made to pass on the sample at glancing angles from 20 to 80 degrees.
The output of the diffraction is obtained as a graph with the counts representing the amount of diffraction.
The FWHM (Full Wave Half Maximum) value (i.e.) the width of the peak at half its height is tabulated along with the D-spacing values.
The values are used for further calculations.
The specifications of the equipment is as follows:
-
Make: PANalytical
-
Model: X’per PRO
-
Source: Cu K (2.2 KW Max.)
-
Detector: X’celerator (Semiconductor)
-
Beta filter: Ni foil (incident beam side) Graphite crystal (diffracted beam side)
-
Attachments sample holder: Sample spinner and zero background sample holder
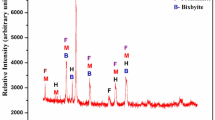
The XRD results are obtained as a graph between the counts and the glancing angle. The graphs and peak values in the graphs for coal ash and the sample 13 ash are shown in Figs. 9 and 10; Tables 5 and 6.
The peaks in the graph show orientation of atoms in a plane at an angle. Though coal has lot of peaks, its maximum count value reaches only to 340.97 which is taken as the reference for relative intensity. However the value of counts reaches a maximum of 1539.06 for sample 13. This denotes high atom orientation in a single lattice plane. Further analysis is to be made and the miller indices, crystal structure and the grain size have to be found out using the JCPDS sheet.
Reproducibility and repeatability
The results of all experiments performed by the author were evaluated with the use of different equipments based on the original experimental description. The results are same with negligible error since the coal mixture could not be affected by weather or any environment condition. The repeatability of results is confirmed by conducting same experiments successively.
Conclusions
The results indicate that the calorific value of the aluminium powder is more than coal. Aluminium powder addition has made to ignite the mixture more quickly. So, it is recommended that aluminium powder is the best alternate to coal. But, the ash content is high and thus the solid waste problems may be expected.
The XRD results show that the crystal structure of the ash of the aluminium powder is having irregular and needle shaped structures. This cannot be used in the boiler parts due to its abrasive effect. So, the solid waste problems may be severe.
However the work will be completed after fulfilling the following:
-
The various types of coal also have to be tested along with aluminium.
-
Since coal arrives at different lots even from the same supplier, different lots of the same coal also have to be tested.
-
Different particle sizes of coal have also to be tested out.
-
Proper waste disposal methods and re-uses for the ash have to be found out.
-
Experimental study of boiler tube abrasion by the ash could be also done.
References
Special article, Metal powder hazards, LPA 4 (2004), 22.
Azhagurajan A. Enhancement of explosive property of nano scale pyro aluminium powder. In: Proceedings international conference on nano materials, MSEC. 2005. ISBN p. 383–87.
Ghosh KN. The principles of fireworks. In: Khastsuria H, editor.; 1987. p. 52.
Lakshmanan K. Chemistry of fire works. In: Proceedings national seminar on Fireworks Safety 99, MSEC: Vikas; 1999. p. 23.
Roth G. Performance of explosives, German patent; No. 1,733,271,900; 2000.
Maranda A. Research on the process of detonation of explosive of mixtures of the oxidizer fuel type containing aluminium powder. Propell Explos Pyrotech. 1990;15:161–65.
Thomas K, Andreas H, Alois K. Influence of aluminium/ammonium perchlorate on the performance of underwater explosives. Propell Explos Pyrotech. 1999;24:140–43.
Gharia JS, Athawale BK, Visal SR, Phadthare VV. Effect of aluminium particle size and percentage on blast compositions, Symposium on Warhead Technology, TBRL: Chandigarh; 1983. p. 144.
Stromose E, Eriksen SW. Performance of high explosives in underwater application, part 2: aluminised explosives. Propell Explos Pyrotech. 1990;15:52–53.
Vinay P. Influence of alumnium on performance of HTPB-based aluminised PBXs. Def Sci J. 2004;4:475.
http://www.argonide.com/propellants.html. Nano aluminum powder for medium-caliber projectiles, accelerator or as a booster.
Mukunda HS. Understanding Combustion, Macmillan India Ltd; 1989. p 22.
Acknowledgements
A. Azhagurajan thanks Head, Department of Mechanical Engineering and Principal, Mepco Schlenk Engineering College for providing financial support to test the chemicals.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Azhagurajan, A., Nagaraj, P. An experimental analysis of coal aluminium mixture in coal fired furnace. J Therm Anal Calorim 98, 253–259 (2009). https://doi.org/10.1007/s10973-009-0009-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0009-4