Abstract
Botswana coal found within the Karoo Basin has received little attention primarily due to limited data on its properties. Several previous researches have been conducted using data for South African coal. However, coal is a heterogeneous material with properties varying not only across different geographic sites but also in iso-seams. Thus, it is important to conduct a study on Botswana coal to determine its combustion characteristics and reactivity. Thermogravimetric analysis was used to study thermal decomposition and determine coal kinetic parameters of coals from the Morupule, Mmamabula and Mabesekwa coalfields. Coal samples were subjected to non-isothermal heating at a heating rate of 25 °C/min in an oxygen atmosphere until a maximum temperature of 1000 °C was reached. Different combustion parameters such as combustion temperatures, and maximum combustion rate were determined from thermogravimetric analysis/derivative thermogravimetric curves. Also determined were the five comprehensive combustion indices for further appreciation of the samples’ combustion traits. Furthermore, the samples were classified based on the chemical composition of the ash. Combustion temperatures were found to be 512.93 ± 3.53 °C to 532.571.36 ± °C ignition temperature, 524.431.17 ± °C to 689.40.56 ± °C peak maximum temperature and 662.771.42 ± °C to 749.73 ± 0.86 °C burnout temperature. Basic oxides in ash could be used to establish the similarities between the coal ash samples via principal component analysis. Proximate-ultimate properties were used to characterize the coal samples into high volatile bituminous and lignite coal. Coal kinetics calculated using pseudo-first-order Arrhenius method yielded activation energies between 42.31 and 60.11 kJ mol−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Botswana, endowed with 212 million tonnes of coal reserves (Paya, 2011), uses coal as its sole natural source for power generation. The success of renewable energy technologies uptake is not yet promising (Ketlogetswe et al., 2007; AUDA-NEPAD, 2016; Zhou, 2016). The country is still striving to penetrate new and renewable energy sources in its energy mix (IRENA, 2021). Although the country is wholly dependent on coal as its source of power, literature on the combustion characteristics of Botswana coal is nonexistent. This presents a challenge when assessing the coal’s technological performance for other coal uses because combustion is a primary process for coal conversion in any technology.
Thermogravimetric analysis was used to study thermal decomposition and determine coal kinetic parameters of coals from the Morupule, Mmamabula and Mabesekwa coalfields. Thermal analysis allows an insight into behavioral characteristics of a material relating to chemical kinetics and mass transport in and out of a particle during a chemical reaction. This information is essential during any coal conversion process because it assists in the selection of the utilization equipment, the utilization technology and the appreciation of coal conversion trait (Silva Filho and Milioli, 2008; Wang et al., 2016; Guo et al., 2018). Over a long period, the perception has been that the process operating conditions take precedence over the nature and properties of coal. However, this led to numerous challenges encountered during the conversion process, indicating that the nature of coal and its characteristics need to be accounted for when selecting coal utilization equipment (Falcon and Ham, 1988). Due to this, comprehensive studies need to be conducted on the combustion performance of coal.
Several studies have been conducted where data from isothermal and non-isothermal experiments were applied on different coal kinetic models to determine coal combustion characteristics such as kinetic parameters, combustion temperatures and combustion performance indices (Rosenvold et al., 1982; Chen et al., 1995; Kok, 2003; Liu et al., 2012; Qi et al., 2017; Song et al., 2017; Engin and Atakül, 2018; Aich et al., 2019; Behera et al., 2019; 2020; Wang et al., 2021). It was noted that proximate properties had an impact on combustion temperatures, and favorable combustion performance could be attained at certain ash and fixed carbon contents while activation energy varied with change in ash content (Aich et al., 2020). Coals with low relative density performed better during combustion than those with high relative density when analyzing changes in chemical properties (Behera et al., 2019). The variations in combustion performance indices and combustion parameters between different relative density fractions were almost linear (Behera et al., 2019).
Although the current study adopted the methodologies from previous studies, the samples used had different proximate properties. They were of high ash, high moisture and medium volatiles. Furthermore, combustion of coal samples was done in a two-stage combustion for better understanding of burning behavior of coal first by breaking the complex organic components of coal into smaller simpler components thereby aid in gaining insights of the factors that influence its burning behavior. Two-stage combustion can be used with a variety of coal types, including low-quality coals, which is the case with studied coal samples. Simple Arrhenius technique assuming first-order Arrhenius kinetics was applied on non-model fitting methods such as the Coats–Redfern and Freeman–Carroll methods, which involve optimizing the kinetic parameters by fitting different models from which kinetic parameters can be calculated. The model-free methods such as the Kissinger, Ozawa and Flynn–Wall methods, on the other hand, utilize different kinetic curves obtained from several heating rates to determine kinetic parameters for the same conversion value (Zhang et al., 2019).
This study investigated the combustion characteristics of coal from the Morupule, Mmamabula and Mabesekwa coalfields in the Kalahari Karoo Basin of the Karoo Super Group. This paper reports first data on the reactivity properties of Botswana coal through the use of the non-isothermal, simple Arrhenius method. Because this was the first study on thermal decomposition and combustion kinetics of Botswana coal, coal samples were burnt using a two-stage combustion process to better appreciate the thermal degradation pattern through the first stage in an inert atmosphere, while the second stage made it possible to determine the combustion kinetics of the coal samples. Several previous researches omitted the first stage (Kaymakçi and Didari, 2002; Liu et al., 2012; Engin and Atakül, 2018; Guo et al., 2018; Behera et al., 2019; Aich et al., 2020; Janković et al., 2020; Zhang et al., 2020; Wang et al., 2021). Also, this study used higher heating rate of 25 K/min as opposed to the convectional 10 K/min. A higher heating rate results in the production of higher proportion of lighter hydrocarbons and less complex aromatic compounds in order to optimize combustion process for improved efficiency and reduced emissions (Strezov et al., 2004; Huangfu et al., 2018). The results of the current study will facilitate future development opportunities of coal resources to be identified by highlighting the coal combustion pattern, which is a primary phase during coal conversion processes.
Methods
Sample Preparation
The Morupule Coal Mine and Shumba (Pty) Ltd responsible for Mabesekwa coal provided fresh samples for this study, while the Botswana Geoscience Institute provided coal cores from the Mmamabula coal mine. Samples from three different sections in each coal mine were investigated, and these were the Morupule East Main (MEM), Morupule West Main (MWM), Morupule South (MS), Mmamabula East (MME), Mmamabula West (MMW), Mmamabula South (MMS), Mabesekwa Seam B (MBB), Mabesekwa Seam C (MBC) and Mabesekwa Seam E (MBE). Only the Morupule Coal Mine is currently operational, and it mines coal for power generation. However, suitable coal utilization technologies are yet to be determined for the other two coal mines. Coal samples were crushed, grinded and sampled according to BIS guidelines, IS 436: Part 1: Sec 1: 1964. Sample particles passing the 72-mesh screen, i.e., < 212 μm, were prepared following the standard sample preparation methods for coal.
Proximate and Ultimate Characteristics
The samples were characterized using proximate and ultimate analyses. Thermogravimetric analyzer and CHN instrumental analyzer were used for determination of proximate and ultimate characteristics as per ASTM D7582 and ISO 12902 guidelines, respectively.
Combustion Characteristics and Kinetics
Thermogravimetric Analysis
Non-isothermal decomposition was conducted in a thermogravimetric analyzer, Mettler Toledo DSC, TGA 3+. The thermogravimetric analyzer was operated at a flow rate of 20 mL/min and 25 °C/min heating rate up to 400 °C in an inert atmosphere. After holding the sample for 10 min at 400 °C, nitrogen gas was substituted with oxygen (0.2 atm) at the same heating rate until the final temperature of 1000 °C was reached. This temperature was maintained until the end of fixed carbon combustion. The reaction regions, combustion temperatures and mass loss were analyzed using STARe thermal software.
Methods of thermogravimetric (TG) measurements were adopted for analyzing the combustion characteristics of the coal samples under study. The combustion parameters depicted in Figure 1 were defined as follows (Liu et al., 2012; Wang et al., 2016; Guo et al., 2018; Aich et al., 2020);
-
i.
Ignition temperature (Ti) was taken as the temperature at which loss of weight is 1 wt.%/min indicating the commencement of the combustion process.
-
ii.
Burn out temperature (Tb) indicates an end to weight loss. At this point, a baseline weight is once again reached.
-
iii.
Peak maximum temperature (Tp) is the temperature at which maximum weight loss occurs.
Combustion Indices
Combustion indices were used for further evaluation of the combustion performance of coal. Ignition index, flammability index, combustion stability index and comprehensive combustibility index indicate the ease or difficulty of coal ignition, how fast residues of char burn, the rate and intensity of combustion process and the overall ignition, combustion and burnout characteristics of coal, respectively.
Combustion indices were calculated using the following equations (Liu et al., 2012; Song et al., 2017; Zhang et al., 2020):
where \(E_{a}\), \(DTG_{\max }\), \(DTG_{{{\text{mean}}}}\) are the activation energy, maximum combustion rate and mean combustion rate, respectively.
Derivation of Kinetic Parameters from Non-isothermal Decomposition of Coal
Kinetic parameters for the determination of the reactivity of coal samples were calculated using first-order simple Arrhenius method following Silva Filho and Milioli (2008). An Arrhenius plot was developed from the thermal decomposition of coal from which the activation energy and Arrhenius constants were determined, thus:
Making k the subject, we have:
Because from the Arrhenius equation, \(k = A\exp^{{ - \frac{{E_{a} }}{RT}}}\), it follows that on substituting in Eq. 6 we get:
Taking natural logs of both sides of Eq. 7, we get
where w, wf, R, T, A and k are initial sample weight, final sample weight, the universal gas constant, temperature, kinetic constant and kinetic reactivity, respectively. Equation 8 follows a linear equation mx + C, thus indicating a linear relationship.
Coal Classification from the Chemical Composition of Ash
Low- and High-Temperature Ashing
The coal samples were subjected to low- and high-temperature ashing. Low-temperature ashing allows for the organic matter to be burnt off while preserving most of the mineral matter, while high-temperature ashing may result in some volatile inorganic matter being lost during the process (Maledi, 2017). For low-temperature ashing, the coal samples were burnt in a Carbolite Gero Laboratory ashing furnace at a heating rate of 50 K/min until a temperature of 400 °C was attained. The burning samples were stirred and mass of sample in the crucible recorded at hourly intervals until there was no mass loss. For high-temperature ashing, the coal samples were burnt in a thermogravimetric analyzer at a heating rate of 25 K/min until a temperature of 900 °C was attained as per ASTM D7582 guidelines.
X-ray Fluorescence Analysis
Coal ash was compressed into pellets using EQP-100 pellet press. The pellets were then transferred to X-Supreme 8000 benchtop material analyzer for determining the composition of ash oxides. The oxides used for principal components analysis (PCA) were MnO2, K2O, SiO2, Al2O3, TiO2, NaO2, CaO, MgO, P2O5, Fe2O3 and SO3.
PCA
Data were collected experimentally. Experimental data regarding coal and coal ash characterization are given in Tables 1 and 3, respectively. Table 3 individual characteristics were used as variables, and PCA was adjusted using SIMCA 16 (Sartorius Stedim Biotech) as previously described in Lima et al. (2022). PCA is a statistical method of great value to environmental samples because of the number of variables we usually characterize in a given model. PCA aims to reduce datasets with many interrelated variables while retaining as much as possible the variation presented in the original data. This reduction is achieved by transforming the actual variables into a new set of variables, the principal components, which are uncorrelated linear combinations of the variables, and that can be visualized. Generally, the distance between a point and the origin (of x and y axes), corresponding to a vector, is proportional to the relative importance of the variable in the PCA model. Points that are close together in a plot, and that their vectors form acute-angled, are significantly correlated—the closer the points are together, the higher the correlation; vectors that form 90° angles imply that variables are not correlated; and variables with vectors that include 180° angles are considered negatively correlated—more details in Jolliffe (2002). The results of the coal characterization are shown in Figure 1, with a two-component PCA model (with a response of 0.789), while results for the coal ash characterization are shown in Figure 4, with a two-component PCA model (with a response of 0.68).
Results Analysis and Discussion
Proximate and Ultimate Characteristics of Coal Samples
The proximate and ultimate properties of coals under study are presented in Table 1. The range of moisture contents was 3.20–7.9%, ash 16.1–64.1 %, volatile matter 15.8–32.2% and fixed carbon 17.6–54.2%, among which the fixed carbon content of the MMS, MBC and MBE coals was very low, while the fixed carbon content of coal in the Morupule coalfield was significantly higher than that of the other samples. Ultimate analysis for all the samples showed that the elemental content of the coal samples was dominated by carbon (C) element, followed by oxygen (O), with hydrogen (H), nitrogen (N) and sulfur (S) accounting for a very small proportion. The content range of C was 22.2–63.3%, H 1.52–3.27%, O 9.12–12.42%, N 0.55–1.45% and S 0.53–1.61%. According to proximate properties, the MEM, MWM and MS coals belong to high-volatile bituminous (HV-B) coal, the MME, MMW and MBB coals belong to lignite (L) while the MMS, MBC and MBE coals could not be classified as coal due to their high ash content. According to the literature (Orem and Finkelman, 2003; Speight, 2015), the ash content in coal does not exceed 50%. Samples with ash content above 50% are representative of coal in its early formation phase (i.e., peat).
From the PCA, we observed that coal samples from the MME were the most different from the remaining ones (Fig. 2b). Although most elements were within a similar range, we observe in Table 1 that the S content of the MME coals was 3-fold of the others. This is critical in terms of incineration settings because high-S coal is among the biggest contributors to sulfur oxide (Sox) anthropogenic emissions (Bodily et al., 1991). The remaining coal types can be grouped mainly into three groups: (1) MEM, MS, MWM; (2) MMW and MBB; and (3) MMS, MBC and MBE. These groups can be roughly explained by their fixed carbon content similarity (Table 1). In fact, fixed carbon, calorific value, hydrogen and nitrogen contents seem to relate quite closely in the PCA loadings (Fig. 2a). This indicates that the characterization methods used relate very closely and can be used interchangeably to classify coal in terms of carbon content. Nevertheless, we recommend to always determine the sulfur content in the coal because it may be an important parameter for incineration performance, and potentially coal ash characteristics.
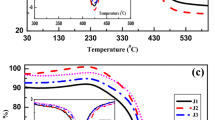
The combustion characteristics concerning the weight loss per reaction phase are summarized in Table 1. The combustion thermograph had demoisturization, devolatilization, combustion and burnout phases, while the temperature profiles and the burning rate from the thermogravimetric analysis/derivative thermogravimetric (TGA/DTG) results are summarized per sample in Table 2. The demoisturization stage accounted for 4.59% minimum weight loss and 5.32% maximum weight loss for the Morupule coalfield; 3.66% minimum weight loss and 5.27% maximum weight loss for the Mmamabula coalfield; 4.8% minimum weight loss and 7.56% maximum weight loss for the Mabesekwa coalfield. After 150 °C, there was a noticeable gain in weight of about 1.2% due to oxygen adsorption by porous coal structure as moisture was removed at low temperatures (Wang et al., 2016; Behera et al., 2019; Aich et al., 2020). The weight loss for the devolatilization stage ranged between 3.71 and 5.51% for the Morupule coalfield; 3.98% and 7.59% for the Mmamabula coalfield; 4.23% and 6.55% for the Mabesekwa coalfield. Most of the weight loss was experienced during the combustion phase with a minimum of 66.39% and a maximum of 73.45% for the Morupule coal field; 29.98% minimum weight loss and maximum of 58.76% for the Mmamabula coalfield; and 29.40% minimum weight loss and maximum of 48.42% for the Mabesekwa coalfield. The high rate of weight loss during combustion by the Morupule coal can be attributed to the increased fixed carbon content (Aich et al., 2020; Wang et al., 2021). The burnout phase gave high weight loss at 5.13% and a minimum of 0.34%. The incombustible remaining material was between 62.04 and 14.45%.
The weight loss per the reaction phases was found to correlate with the proximate properties of the samples. The maximum weight loss per the reaction phases was found to correspond with the maximum content value of the proximate parameters. The minimum weight loss corresponded with the minimum content value of the proximate parameters. Rosenvold et al. (1982) also reached the same conclusion; they noted that thermal decomposition of coal through TGA yielded results in agreement with ASTM values.
Thermogravimetric Analysis
The obtained TGA/DTG results are shown in Figure 4. After 500 °C, rapid loss of weight is seen, indicating the commencement of coal combustion. The Ti ranged 529.0–532.57 °C for the Morupule coal field, 512.93–524.57 °C for the Mmamabula coalfield and 520.27–522.57 °C for the Mabesekwa coalfield. The different values of ignition temperature for coal samples from the same coalfield can be attributed to their different proximate properties in particular volatile matter content (Silva Filho and Milioli, 2008; Wang et al., 2016; Aich et al., 2020). The Tp ranged 541.53–689.40 °C for the Morupule coalfield, 535.43–560.90 °C for the Mmamabula coalfield and 524.53–535.40 °C for the Mabesekwa coalfield. The corresponding peak burning rates were 0.84–2.27 wt.%/min for the Morupule coalfield, 1.92–3.10 wt.%/min for the Mmamabula coalfield and 2.80–3.11 wt.%/min for the Mabesekwa coalfield. A high maximum combustion rate (DTGmax) indicates easier diffusion of oxygen/carbon dioxide into coal particles (Behera et al., 2019). The high Tp for the MEM coal is due to increased carbon content and low ash content, commonly a trait of high-rank coals resulting in enhanced calorific value. Toward the end of the non-isothermal testing, the samples displayed constant weight, indicating the end of combustion and hence the generation of ash. The burnout temperatures were 697.70–749.73 °C for the Morupule coalfield, 681.70–693.70 °C for the Mmamabula coalfield and 662.77–710.43 °C for the Mabesekwa coalfield. The high burnout temperature displayed by the Morupule coal indicates that it burns at a lower rate taking a long time for combustion to complete. High burnout temperature signifies that coal has high resistance to oxygen reaction and thus the heat release rate is lowered, which can result in residual unburnt carbon should the coal be burnt at low temperatures (Liu et al., 2012; Aich et al., 2020).
The DSC profile reveals the type of reaction that occurred (Fig. 3c, f, i). All the samples displayed predominant heterogeneous combustion verified by a sharp peak in the exothermic reaction phase. The presence of a single peak indicates smooth burning of coal stage after another through moisture elimination, hydrocarbons release and elimination of high molecular weight hydrocarbons (Aich et al., 2019). However, the samples from the MEM had two distinct heterogeneous combustion phases around 400 °C and 600 °C, indicating stage by stage combustion of coal as hydrocarbons were released during different stages. This type of combustion can be attributed to existing hydrocarbons with varying molecular weight structures within the coal structure (Kok, 2003; Aich et al., 2020). The first heterogeneous combustion is due to the devolatilization of lower molecular weight structures before the combustion of high molecular weight structures.
Analysis of Combustion Indices
Further combustion of coal was studied by analyzing different combustion indices (Table 2).
The Di for the samples varied from 2.01 × 10−6 to 8.39 × 10−6 wt.%/min4 for all the samples. Coal with low Di will experience increased difficulty during burning, while high index yields increased combustion. The higher the Di, the more smoothly coal will burn (Song et al., 2017; Aich et al., 2020; Zhang et al., 2020). Some of the samples displayed high Dc as compared to previous studies (Aich et al, 2020), which could be attributed to high ash content, thus more oxides are present resulting in an increased overall combustion index. Coals with increased Dc possess better combustion characteristics (Guo et al., 2018; Zhang et al., 2020).
The flammability index, C, was above 5 × 10−6 wt.%/min4 except for the MEM, indicating that coal from this section possesses poor combustion properties. Coal with high flammability index has high capacity for combustion (Song et al., 2017). The Hf ranged 2.32–5.83 °C. Because the smaller the Hf, the better coal combustion performance is, coal from the MMS with low Hf has better combustion performance than all the others, while that from the MEM will have poor combustion performance (Qi et al., 2017; Song et al., 2017; Behera et al., 2019; Aich et al., 2020). The poor combustion performance predicted for the MEM could be due to several factors. Aich et al. (2020) revealed that the fixed carbon content ideal for good combustion performance lies in the 28–39% range. Therefore, the fixed carbon content of the MEM might have negatively impacted on its combustion performance. Although the fixed carbon content for the MWM and MS was outside the stated range, their combustion performance was better compared to the MEM, and this can be attributed to the ash content and amount of hydrocarbons present in coal. When there are more hydrocarbons present, there is less ash content, the overall O2/CO2 transport slows down reducing the rate of heat release (Aich et al, 2020). The composition of organic matter is also an important factor during combustion as they react differently. Vitrinite has less hydrogen and more oxygen yielding char with high porous structure (hence, it is more reactive), whereas inertinite is low in volatile matter but rich in aromatic carbons rendering it the least reactive, and liptinite has high yields of volatiles and hence it is the most reactive (Suarez-Ruiz, 2012). Any of these factors might have led to poor combustion performance predicted for the MEM because the coal samples indicated properties of higher rank than all the samples under study and hence it is less reactive.
Coal Ash Classification using Chemical Composition of Ash
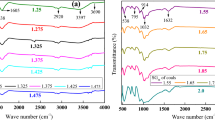
Basic oxides (Table 3) that make up the chemical composition of ash were used to classify coal ash using PCA (Fig. 5). Four clusters were identified, one in each quartile from the PCA diagram. The first comprised of NaO2, Al2O3, SiO2; the second had K2O, TiO2; the third comprised of CaO, MnO3; and the fourth was made up of MgO, P2O5 and SO3. The results indicate that the components per cluster were strongly correlated. The correlation signifies that data on any of the variables in the clusters can be used to determine the similarities of ash from the samples under study.
The loaded variables resulted in the scores that were used to classify coal ash (Fig. 4). Analysis was conducted for both high- and low-temperature ash. The results for high- and low-temperature ash display very close correlations for all the samples, indicating that ash from any of those temperatures will yield the same results when used for classification.
Three clusters were identified from the scores. The first cluster appearing in the first quartile comprises the MEM, MWM and MS coals; the second clusters in the second quartile consists of the MMW, MMS, MBB, MBC and MBE coals while ash from the MME was separated from all the other samples in the fourth quartile. The clusters indicate similar characteristics of ashes from those coalfields. Coal ash from coal samples in the first cluster can be characterized by Al2O3, NaO2 or SiO2 content, while coal ash from coal samples in the second cluster can be characterized either by the content of K2O or TiO2. Coal ash obtained from combustion of the MME coal samples can be characterized by P2O5, SO3, CaO, Mn2O3, Fe2O3 or MgO content.
Thermogravimetric Kinetic Characteristics
Kinetic Parameters
The calculated reaction kinetics, activation energy (Ea) and kinetic constant (A) are as shown in Table 1. Figure 5 shows the Arrhenius plots that were used to calculate the reaction kinetic parameters. All the nine samples displayed similar reaction patterns consisting of primary combustion (combustion of volatiles) and secondary combustion (combustion of fixed carbon) (Fig. 5a). The activation energies for the nine samples ranged 42.31–60.11 kJ mol−1. The MEM had the highest activation energy among all the samples, while the MBE sample displayed the least. The high activation energy signifies that samples from the MEM require more energy to overcome the bonds between the structural atoms. This activation energy is in agreement with its overall proximate and ultimate characteristics and combustion performance. Samples from the MEM with higher fixed carbon content, lower ash content and high carbon content are from a parent rock of higher rank compared to all the other samples.
Furthermore, the high ignition temperature, high peak maximum temperature, low maximum combustion rate and low comprehensive performance index signify coal of high rank. The MMW, MBB and MBC coals had almost similar activation energies. However, their proximate and ultimate properties are different. These can be attributed to other coal properties such as, among others, maceral and chemical structural composition, and particle pore structure. The attained correlation for the kinetic parameters shown by the R-squared value greater than 0.7 is satisfactory. Onifade and Genc (2018), when predicting spontaneous combustion, attained a 0.812 correlation factor, which is close to the one attained in the current study.
Comparison of Activation Energy with Literature
The activation energies from the present study are within the range of activation energies reported in the literature (Table 4). Regardless of the dissimilarities in properties of the coal samples under study, Wang et al. (2010), Saloojee (2011) obtained activation energies in the range within, which activation energies from this current study fell. Thus, validating findings from previous studies that coal reactivity is not dependent only on coal type. Kinetic modeling and operational conditions such as heating rates also have a contribution.
Comparison of Combustion Temperatures, Combustion Indices and Kinetic Parameters
A two-factor ANOVA with replication was applied for the comparison of combustion temperatures, combustion indices, kinetic parameters and the coalfields, while one-factor ANOVA was applied for comparison among the coalfields.
The hypothesis for two-factor ANOVA testing are:
H1
The means of observations grouped by combustion temperatures or indices are the same
H2
The means of observations grouped by coalfields are the same.
The hypotheses for one factor ANOVA testing are
H1
The true mean for the coalfields is the same (i.e., the coalfields are comparable)
H2
There is at least one inequality in the coalfields.
The results from the two-factor ANOVA indicate that the means of observations grouped by combustion temperatures and combustion indices are not the same (Table 5). The F-values, 304.78 and 123.17, were greater that the F-critical values of 3.01 and 2.69, respectively, for combustion temperatures and combustion indices, respectively. These mean that there is a significant difference in at-least two parameters in the groups; hence, comparing between either the combustion temperatures or combustion indices will be invalid. The conclusion is also substantiated by the P value, which is less than the alpha set at 5%. The means of observations grouped by the coalfields was found to be the same (Table 5). The F-values, 3.12 and 2.69, were less than the F-critical values of 3.40 and 3.32, respectively, for combustion temperatures and combustion indices, respectively. Also, the P value was greater than the alpha, an indication that comparison of the coalfields was acceptable. The means of observations for both the kinetic parameters and coalfields was found not to be true (Table 5). However, a comparison could be made for the MMW, MBC and MBE coals using a one factor ANOVA testing (Table 6). The results of the statistical analysis signify that the analysis of the investigated combustion parameters, which was done by comparing the coalfields rather than the parameters, was correct.
Conclusions
Non-isothermal thermogravimetric analysis was conducted on nine samples from three different coal fields. Proximate and ultimate analyses were used to characterize the samples. The samples displayed a similar combustion pattern with ignition temperatures of 512.93–532.57 °C, peak maximum temperatures of 524.43–689.40 °C, burnout temperatures of 662.77–749.73 °C and maximum combustion rates of 0.84–3.11 wt.%/min. The following conclusions were made relating to the classification of coal and coal ash basing on proximate-ultimate properties and chemical composition of ash, respectively.
-
The MEM, MWM, MS coals were classified as high volatile bituminous coal with volatile matter contents of 19.8–24.3%, fixed carbon contents of 51–54.2%; ash contents of 19.0–24.4% and carbon contents of 56.1–63.3%.
-
The MME, MMW and MBB coals were classified as lignite coal with volatile matter contents of 20.2–32.2%, fixed carbon contents of 35.3–38.9%, ash contents of 23.7–36.3% and carbon contents of 40.9–48.9%.
-
The MMS, MBC and MBE coals, due to their high ash content, could not be classified as coal. Coal samples from these coalfield sections had ash contents of 56.3–64.1%.
-
Coal ash from the MEM, MS and MWM coal samples could be characterized by Al2O3, NaO2 or SiO2 contents.
-
Coal ash from the MMS, MMW, MBB, MBC and MBE coal samples could be characterized by either K2O or TiO2 content.
-
Coal ash from the MME coal samples could be characterized by P2O5, SO3, CaO, Mn2O3, Fe2O3 or MgO content.
The first-order Arrhenius method was successful capturing the reaction kinetics. Activation energies attained varied between 42.31 and 60.11 kJ mol−1.
References
Aich, S., Behera, D., Nandi, B. K., & Bhattacharya, S. (2020). Relationship between proximate analysis parameters and combustion behaviour of high ash Indian coal. International Journal of Coal Science & Technology, 7(4), 766–777.
Aich, S., Nandi, B. K., & Bhattacharya, S. (2019). Utilization of sal leaves and sal leaves char to improve the combustion performance of reject coal. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 41(19), 2299–2312.
AUDA-NEPAD. (2016). Rapid assessment and gap analysis: Botswana. https://www.se4all-africa.org/fileadmin/uploads/se4all/Documents/Country_RAGAs/Botswana-Rapid-assessment-Gap-Analysis-Final.p
Behera, D., Nandi, B. K., & Bhattacharya, S. (2019). Chemical properties and combustion behavior of constituent relative density fraction of a thermal coal. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 41(6), 654–664.
Bodily, D. M., Wann, J.-P., Chen, W., Zhu, X., Hu, W., & Wadsworth, M. E. (1991). Characterization of mineral and coal pyrite (B. T.-1991 I. C. on C. S. P. International Energy Agency Coal Research Ltd (ed.); pp. 973–976). Butterworth-Heinemann. https://doi.org/10.1016/B978-0-7506-0387-4.50246-1
Chen, Y., Mori, S., & Pan, W.-P. (1995). Estimating the combustibility of various coals by TG-DTA. Energy and Fuels, 9(1), 71–74.
da Silva Filho, C. G., & Milioli, F. E. (2008). A thermogravimetric analysis of the combustion of a Brazilian mineral coal. Química Nova, 31(1), 98–103.
Engin, B., & Atakül, H. (2018). Air and oxy-fuel combustion kinetics of low rank lignites. Journal of the Energy Institute, 91(2), 311–322.
Falcon, R., & Ham, A. J. (1988). The characteristics of Southern African coals. Journal of the South African Institute of Mining and Metallurgy, 88, 145–161.
Guo, L., Zhai, M., Wang, Z., Zhang, Y., & Dong, P. (2018). Comparison of bituminous coal and lignite during combustion: Combustion performance, coking and slagging characteristics. Journal of the Energy Institute, 92(3), 802–812.
Huangfu, W., You, F., Shao, Y., Wang, Z., & Zhu, Y. (2018). Effects of oxygen concentrations and heating rates on non-isothermal combustion properties of jet coal in East China. Procedia Engineering, 211, 262–270.
IRENA. (2021). Renewables readiness assessment: Botswana. www.irena.org/publications
Janković, B., Manić, N., Stojiljković, D., & Jovanović, V. (2020). The assessment of spontaneous ignition potential of coals using TGA–DTG technique. Combustion and Flame, 211, 32–43.
Jolliffe, I. T. (2002). Principal component analysis. Springer-Verlag. https://doi.org/10.1007/0-387-22440-8_1
Kaymakçi, E., & Didari, V. (2002). Relations between coal properties and spontaneous combustion parameters. Turkish Journal of Engineering and Environmental Sciences, 26, 59–64.
Ketlogetswe, C., Mothudi, T. H., & Mothibi, J. (2007). Effectiveness of Botswana’s policy on rural electrification. Energy Policy, 35, 1330–1337.
Kok, M. V. (2003). Fossil fuels: Application of thermal analysis techniques. In M. E. Brown & P. K. Gallagher (Eds.), Applications to inorganic and miscellaneous materials (pp. 371–395). Elsiver.
Lima, A. T., Kirkelund, G. M., Ntuli, F., & Ottosen, L. M. (2022). Screening dilute sources of rare earth elements for their circular recovery. Journal of Geochemical Exploration, 238, 107000.
Liu, Y., Wang, C., & Che, D. (2012). Ignition and kinetics analysis of coal combustion in low oxygen concentration. Energy Sources Part A-Recovery Utilization and Environmental Effects, 34, 810–819.
Maledi, N. B. (2017). Characterisation of mineral matter in South African coals using micro-raman spectroscopy and other techniques [University of the Witwatersrand, Johannesburg]. https://hdl.handle.net/10539/24090
Onifade, M., & Genc, B. (2018). Prediction of the spontaneous combustion liability of coals and coal shales using statistical analysis. Journal of the Southern African Institute of Mining and Metallurgy, 118, 799–808.
Orem, W. H., & Finkelman, R. B. (2003). Coal formation and geochemistry. In F. T. Mackenzie (Ed.), Sediments, diagenesis, and sedimentary rocks: Treatise on geochemistry (Vol. 7, pp. 191–222). Elsevier-Pergamon.
Paya, B. (2011). The coal road map pitso-An overview of Botswana’s resources and future plans.
Qi, X., Li, Q., Zhang, H., & Xin, H. (2017). Thermodynamic characteristics of coal reaction under low oxygen concentration conditions. Journal of the Energy Institute, 90(4), 544–555.
Rosenvold, R. J., Dubow, J. B., & Rajeshwar, K. (1982). Thermal analyses of Ohio bituminous coals. Thermochimica Acta, 53(3), 321–332.
Saloojee, F. (2011). Kinetics of pyrolysis and combustion of a South African coal using the distributed activation energy model. University of the Witwatersrand.
Song, C.-Z., Wen, J.-H., Li, Y.-Y., Dan, H., Shi, X.-Y., & Xin, S. (2017). Thermogravimetric assessment of combustion characteristics of blends of lignite coals with coal gangue. In Proceedings of the 3rd annual international conference on mechanics and mechanical engineering (MME 2016), 490–495.
Speight, J. G. (2015). Handbook of coal analysis: Proximate analysis. https://doi.org/10.1002/9781119037699.ch5
Strezov, V., Lucas, J. A., Evans, T., & Strezov, L. (2004). Effect of heating rate on the thermal properties and devolatilisation of coal. Journal of Thermal Analysis and Calorimetry, 78, 385–397.
Suarez-Ruiz, I. (2012). Organic petrology: An overview. In A. Al-Juboury (Ed.), Petrology—new perspectives and applications (pp. 199–224). Intech. https://doi.org/10.5772/23431
Wang, C., Wang, C., Jia, X., Gao, X., Wang, P., Feng, Q., & Che, D. (2021). Experimental investigation on combustion characteristics and kinetics during co-firing bituminous coal with ultra-low volatile carbon-based solid fuels. Journal of the Energy Institute, 95, 87–100.
Wang, J.-H., Chang, L.-P., Li, F., & Xie, K.-C. (2010). A study on the combustion properties of western chinese coals. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 32(11), 1040–1051.
Wang, Y., Song, Y., Keduan, Z., Li, Y., Teng, Y., He, R., & Liu, Q. (2016). Combustion kinetics of Chinese Shenhua raw coal and its pyrolysis carbocoal. Journal of the Energy Institute, 90, 624–633.
Zhang, H., Dou, B., Li, J., Zhao, L., & Wu, K. (2020). Thermogravimetric kinetics on catalytic combustion of bituminous coal. Journal of the Energy Institute, 93(6), 2526–2535.
Zhang, Q., Luo, M., Yan, L., Yang, A., & Hui, X. (2019). Kinetic analysis of low-rank coal pyrolysis by model-free and model-fitting methods. Journal of Chemistry, 2019, 9075862.
Zhou, P. P. (2016). Development and energy in Africa(DEA)project: A case study for Botswana-Rural Electrification by Grid Electrification.
Acknowledgments
The authors would like to thank the management of Botswana International University of Science and Technology for financing this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
A declaration is made that no competing financial interests nor personal relationships exist that could have appeared to influence the work reported in this paper.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Keboletse, K.P., Ntuli, F., Oladijo, O.P. et al. Comprehensive Analysis of Coal Combustion Characteristics and Kinetic Parameters of Botswana Coal, Morupule, Mmamabula and Mabesekwa Coalfields. Nat Resour Res 32, 2805–2819 (2023). https://doi.org/10.1007/s11053-023-10254-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11053-023-10254-9