Abstract
A new formulation of nifedipine tablets was prepared. The tablets were conditioned in amber-colored glass containers and placed in a climatized room at 40 °C and relative humidity of 75% for 180 days. Differential scanning calorimetry (DSC) and Thermogravimetry (TG) were used in order to evaluate the thermal properties of nifedipine, the excipients and two well-known nifedipine degradation products. There is no evidence of interaction between nifedipine and excipients or degradation products. High performance liquid chromatography (HPLC) was used in the dosage of nifedipine tablets before and after acclimatized exposure. Results show that DSC and TG offer important data for a more detailed assessment of the stability of a pharmaceutical formulation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Drugs are rarely administrated in their isolated form. Most of them are related to a variety of adjuvants and excipients (diluents, suspensors, lubrificants desintegrants) in pharmaceutical formulations, which are submitted to technological process, with major or minor sophistications, that makes possible to obtain stable and biodisponible pharmaceutical forms, allowing a precise dosage and defined posology, that are reproducible in agreement with a planed administration way [1].
Nifedipine is a pharmaceutical blocking agent of calcium channels used in the treatment of systemic arterial hypertension [2]. It reveals an effective antihypertensive which is similar to that of diuretics, beta-adrenergic blockers and angiotensin conversion enzyme inhibitors [3, 4]. Nifedipine, (1,4-dihydro-2,6-dimethyl-4-(2-nitrophenyl)-3-5-pyridinedicarboxylic acid dimethyl ester) [5] may suffer alterations due to light exposure [6, 7]. According to Merck Index its melting point varies between 172 and 174 °C [8].
In the development of a new pharmaceutical formulation, is important to identify the interaction among the components based in their physical and chemical characteristics. This evaluation can be used to prevent the occurrence of future stability problems. Differential scanning calorimetry (DSC) and thermogravimetry (TG) provides important data on the evaluation of stability of a pharmaceutical formulation [9]. Interactions between pharmaceutical drugs and excipients may generate several compatibility problems which affects the stability of the formulation [10]. DSC is systematically employed for the compatibility studies between pharmaceutical drugs and excipients with success as it can be concluded from the large number of published papers in this field. Kiss et al. [11] studied the compatibility between metronidazol and different excipients and detecting interaction between metronidazol and calcium diphosphate dehydrate. Stulzer et al. [12] investigated the compatibility between captopril and pharmaceutical excipients used in tablets formulations. The DSC curves revealed a possible drug-excipient interaction with magnesium stearate. Vlase et al. [13] studied the thermal stability of some phenitoine pharmaceuticals and the research has shows the kinetics of decomposition of this drug.
Abbas et al. [14] investigated the properties of a substituted pyridoquinoline and its interaction study with excipients and the results showed that pyridoquinoline is a very stable compound and compatible with several pharmaceutical excipients. The work of Santos et al. [15] investigate the possible interactions between metformin and excipients as microcrystalline cellulose, sodium croscarmellose, PVP K30, magnesium stearate, starch and lactose, usually employed in pharmaceutical products. Thermogravimetric studies (TG) of metformin and its binary mixtures showed different thermal behavior. Filho et al. [16] investigated the possible interactions between nifedipine and excipients as microcrystalline cellulose, magnesium stearate, silicon dioxide and hydroxipropilmethylcellulose and demonstrated that there is no evidence on the interaction between nifedipine and excipients, or degradation products.
The novelty of the present study is to evaluate the compatibility between nifedipine and some pharmaceutical excipients as starch and lactose, employed in pharmaceutical products.
Materials and methods
Materials
-
nifedipine (batch 406059, Zambon), nifedipine USP reference standard (batch KOD401), nifedipine nitrophenylpyridine USP standard (batch K46360), and nifedipine nitrosophenylpyridine USP standard (batch K46370) as drug and
-
starch (batch 3014289197, Corn P. Brasil), monohydrate lactose (batch 416027, Frieslant), microcrystalline cellulose (batch 103, Reliance), magnesium stearate (batch MGSV 40372, Faci), silicon dioxide (batch 20704/2, Cabot), hydroxypropylmethylcellulose 4.000—hpmc 4 M (batch PJ 29012N02, Colorcon) as excipients have been investigated.
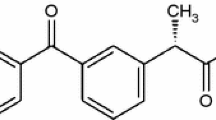
The chemical structure of nifedipine and its degradation products are represented in the Fig. 1
Methods
Preparation of formulation
Tablets containing 20 mg of nifedipine were prepared in a light-protected environment by direct compression in a Lawes compressor (model 10) and a group of matrices and biconcave punctures with 6 mm in diameter were used. The constituents in the formulation were: nifedipine, starch, monohydrated lactose, microcrystalline cellulose, hpmc 4 M, magnesium stearate, silicon dioxide.
Storage experiments
Tablets were put into an amber-colored glass and placed for 180 days in a special acclimatized room which was equipped with a Mecalor air conditioner operating at 40 (±2 °C) and the relative humidity was kept at 75 (±5%) [17].
Dosage
The amount of nifedipine in the tablets was determined via high performance liquid chromatography (HPLC) according to [6] using PerkinElmer equipment which possessed a UV/VIS detector. The experimental conditions in HPLC were: detector of 265 nm and an analytical column of 4.6 mm × 25 cm containing packaging type L1 and a guard column of 2.1 mm × 3 cm, containing packaging L1. The flow rate is approximately 1.5 mL per minute.
Thermal analytical tests
Nifedipine, USP standards, excipients and tablets before and after storage were measured using differential scanning calorimetry (DSC), Mettler Toledo 822e at atmosphere of synthetic air, with a flow rate of 50.0 mL min−1, and at a heating rate of 10 °C min−1, between 25 and 350 °C using aluminium crucible with a perforated lid cover. The initial mass of nifedipine and excipients was around 5 mg. The initial mass of reference standards—nifedipine nitrophenylpyridine USP, nifedipine nitrosophenylpyridine USP and nifedipine USP—was around 1 mg. For the tablet analysis ten pieces were used with an initial mass of about 5 mg. Check of indium was made for standardization.
Thermogravimetric curves (TG) were obtained by the aid of Mettler Toledo TG/SDTA 851e equipment at synthetic air atmosphere (flow rate: 50.0 mL min−1, heating rate of 10 °C min−1) using alumina crucible in the 25–800 °C temperature range. The initial sample masses were around 6 mg. A blank curve and check of indium and aluminum were made for standardization.
Results and discussion
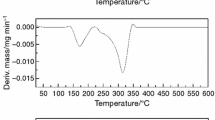
Figure 2 shows the TG curves of nifedipine and the pure excipients are collected while Fig. 3 shows the thermogravimetric curves of the tablets before and after storage. Their thermal decomposition ranges are summarized in Table 1.
Magnesium stearate is thermally stable up to 290 °C. Microcrystalline cellulose is thermally stable up to 285 °C, and hpmc 4 M is thermally stable up to 240 °C. Silicon dioxide does not decompose in the temperature range under analysis. Starch is thermally stable up to 278 °C. The TG curve from lactose (Fig. 1) shows the first step of mass loss (5% mass) that occurs from 106 to 164 °C, this step was attributed to the dehydration of the molecule. The empirical formula of lactose monohydrate is C12H22O11. H2O and its molecular mass is equal to 360.31 g/mol [18]. The percentage of linked water will be given by the ratio: %H2O = 18.0/360.31 * 100 = 5.0%. The second step is observed from 216 to 339 °C (66% mass loss). Nifedipine is thermally stable up to 210 °C. The TG curves of the tablets before and after storage show that the formulation is thermally stable up to 143 °C which corresponds well to the initial decomposition temperature of monohydrate lactose.
Figure 4 shows the DSC curves of nifedipine and excipients compared to the DSC curves of the tablets before and after 180 days of storage.
DSC curves of magnesium stearate, cellulose, HPMC 4 M and starch are initially exhibit wide endotherm representing dehydration starting around 40–50 °C. The DSC curves of the tables show also wide endotherm around 45 °C due to dehydration of these three excipients. Magnesium stearate reveals a wide endotherm peak between 45 and 90 °C followed by another endotherm one up to 109 °C. This second peak is not present in the DSC curves of the tablets probably due to the low amount of this excipient in the formulation. Silicon dioxide does not present any thermal event in the investigated temperature range. The DSC curve from lactose monohydrate shows two endothermic peaks. The first peak with a maximum at 145 °C corresponds to water loss. The second peak, with maximum at 217 °C is attributed to the fusion of anhydrous α-lactose [18].
The DSC curve of tablet before storage (Fig. 3, a) shows the fusion of nifedipine takes place between 170 and 176 °C showing no alteration compared to the melting range when nifedipine is alone. The similar thermal behavior of nifedipine alone and in its tablet denies the occurrence of any chemical interaction between nifedipine and the excipients upon tablet making as well as during its storage which is supported by the results of the HPLC analysis of tablets (Fig. 5) as well.
Besides the observed retention time (around 15 min) the result of the nifedipine content determination in the tablet before storage was 95.4% (m/m) and after storage was 91.0% (m/m).
Figure 6 summarizes the DSC curves of nifedipine and its degradation products, nifedipine nitrophenylpyridine, nifedipine nitrosophenylpyridine and the DSC curves of the tablets both before and after storage.
In the DSC curves endotherm peaks are visible between 103 and 108 °C for nifedipine nitrophenylpyridine and between 93 and 98 °C for nifedipine nitrosophenylpyridine. Since these peaks are not in present in the DSC curves of the tables, it can be concluded that no visible amount of degradation product can be present in these formulates.
Conclusions
To certify the quality of a pharmaceutical formulation, some parameters must be analyzed, such the interaction between this drug and the formulations’ with excipients.
The developed formulation can be considered as stable ones. There were no signs of interaction between nifedipine and excipients, which supports the stability of the pharmaceutical drug in its formulation and after storage the tablets. Finally, presence of the representative degradation products of nifedipine was not detected by DSC methods.
References
Aulton ME. Pharmaceutics the science of dosage form design. 6th ed. New York: Churchill Livingstone; 1996. p. 1–13.
Rang HP, Dale MM, Ritter JM. Farmacologia, Guanabara Koogan. 1997; p. 234.
Reid JL. First-line and combination treatment for hypertension. Am J Med. 1989;86(4A):2–5.
Man in’t Veld AJ. Calcium antagonists in hypertension. Am J Med. 1989;86(4A):6–14.
Martindale, The extra pharmacopeia. 30 edn. London: The Pharmaceutical Press; 1993. p. 374.
United States Pharmacopeia and National Formulary, 2005; p. 1377–1379.
Trissel LA. Stability of compounded formulations. Washington: Amer Pharm Assoc; 2000. p. 440–50.
The Merck Index. 20th edn. 1996; p. 6620.
Giron D. Applications of thermal analysis and coupled techniques in pharmaceutical industry. J Therm Anal Calorim. 2002;68:335–57.
Drebushchak VA, Shakhtshneider TP, Apenina SA, Medvedeva AS, Safronova LP, Boldyrev VV. Thermoanalytical investigation of drug–excipient interaction. Part II. Activated mixtures of piroxicam with cellulose and chitosan. J Therm Anal Calorim. 2006;86:303–9.
Kiss D, Zelkó R, Novák Cs, Éhen Zs. Application of DSC and NIRS to study the compatibility of metronidazole with different pharmaceutical excipients. J Therm Anal Calorim. 2006;84:447–51.
Stulzer HK, Rodrigues PO, Cardoso TM, Matos JSR, Silva MAS. Compatibility studies between captopril and pharmaceutical excipients used in tablets formulations. J Therm Anal Calorim. 2008;91(1):323–8.
Vlase G, Vlase T, Doca N. Thermal behavior of some phenitoine pharmaceuticals. J Therm Anal Calorim. 2008;92(1):259–62.
Abbas D, Kaloustian J, Orneto C, Piccerelle P, Portugal H, Nicolay A. DSC and physico-chemical properties of a substituted pyridoquinoline and its interaction study with excipients. J Therm Anal Calorim. 2008;93(2):353–60.
Oliveira Santos AF, Basílio ID Jr, de Souza FS, Medeiros AFD, Pinto MF, de Santana DP, et al. Application of thermal analysis in study of binary mixtures with metformin. J Therm Anal Calorim. 2008;93(2):361–4.
Filho ROC, Franco PIBM, Conceição EC, Leles MIG. Stability studies on nifedipine tablets using thermogravimetry and differential scanning calorimetry. J Therm Anal Calorim. 2008;93(2):381–5.
Resolução RE nº 1, de 29 de julho de 2005 da ANVISA. Publicado no D.O.U. – Diário Oficial da União; Poder Executivo, de 01 de agosto de 2005.
Handbook of Pharmaceutical Excipients. The Pharm. Press, London and Amer. Pharm. Association, Washington; 1994.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Filho, R.O.C., Franco, P.I.B.M., Conceição, E.C. et al. Stability studies on nifedipine tablets using thermogravimetry and differential scanning calorimetry. J Therm Anal Calorim 97, 343–347 (2009). https://doi.org/10.1007/s10973-009-0083-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0083-7