Abstract
This work presents the preparation and characterization of magnesium ferrite which is one of the important magnetic oxides with spinel structure. Magnesium ferrite was prepared via microemulsion method mediated hydrolytic decomposition of mixed alkoxide solutions. This microemulsion was using for preparation magnesium ferrit for the first time. The starting solution, composed from magnesium methoxide and iron ethoxide in dry ethanol, was introduced in to the prepared microemulsion and sequentially hydrolyzed by distilled water addition (Pithan et al. in J Cryst Growth 280:191–200, 2005; Shiratori et al. in J Eur Ceram Soc 25:2075–2079, 2005; Herrig and Hempelmann in Mater Lett 27:287–292, 1996). After raw powder precipitation, the samples were decantanted by ethanol and then calcined at temperatures 800, 900, 1,000 or 1,100 °C for 1 h. The resulting samples were characterized using powder X-ray diffraction, high resolution transmission electron microscopy, Mössbauer spectroscopy and magnetic measurements. X-ray diffraction and Mössbauer spectroscopy confirmed the presence of the spinel phase. The particles size was calculated from the XRD line broadening using Scherrer equation and their size was found about 31–38 nm, with only slight dependence on the heat treatment temperature. TEM revealed the particles size of about 39 nm. Magnetic measurements showed a ferrimagnetic behavior for all samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Magnetic particles have become a subject of considerable interest in last few decades and many physical studies have been devoted of them. The ability to produce nano-sized magnetic materials opened new application for magnetic materials, such as magnetic data storage, magnetocaloric refrigeration, electronics, ferrofluid technology, magnetically targeted drugs carriers, contrast agents in magnetic resonance imaging, etc.
As is known, some magnetic properties such as saturation magnetization, remanent magnetization and coercivity depend strongly on the particle size and microstructure of the materials. Therefore, it is interesting and important to develop techniques by which the size and shape of particles can be well controlled. One of the ways to prepare the nanocomposites with the required properties represents microemulsion method. Principle of this method is hydrolysis of alkoxide solution, which is encapsulated in microdroplets of the microemulsion. Separate microdroplets act as separate “microreactors” with specific size and narrow size distribution. Final particle size is determined by the microdroplet size. Main advantage of this method is obtention of pure nanomaterial without any matrix and relatively low preparation temperatures. On the other side, main disadvantage of this method are synthesis under inert atmosphere and bad commercial accessibility of some alkoxides.
In this paper, we present the synthesis of MgFe2O4 nanoparticles and their characterization by magnetic measurements, Mössbauer spectroscopy, X-ray diffraction and high resolution transmission electron microscopy. Magnesium ferrite has spinel structure, generally denoted by the formula AB2O4, where the A-ions occupy tetrahedral sites and the B-ions octahedral sites of the lattice. Magnesium ferrite has spinel structure, where magnesium and iron ions occupies in the tetrahedral A-sites and in the octahedral B-sites of the spinel. Spinel are often known that the ratio of the number of atoms in the octahedral and in the tetrahedral cavity differ from the ideal state. For this reason, it can be expected relative deviations from the theoretical sextet area ratio 1:2 (tetrahedral sites:octahedral sites).
Second purpose of this work is confirmation of the microemulsion method suitability for preparation different ferrite nanoparticles (cobalt ferrite, zinc ferrite, manganese ferrite, etc.).
2 Experimental
2.1 Sample preparation
The raw nanopowder was prepared through the hydrolysis of microemulsion containing alkoxides solution by water addition. The starting alkoxide solutions were prepared from magnesium methoxide and iron ethoxide (although other alkoxides could also be used) in dry ethanol. Starting magnesium methoxide was used commercial (Sigma–Aldrich). Starting iron ethoxide was obtained by reaction of sodium ethoxide with iron trichloride in dry ethanol under dry atmosphere. Sodium ethoxide was prepared from metal sodium and dry ethanol by standard procedure [4]. Stoichiometric mixture of alkoxide solutions was dropwise added to prepared microemulsion (Lutensol ON 110 10.06 wt%, cyclohexane 81.36 wt%, 1-octanol 5.94 wt%, and distilled water 2.64 wt%). After thorough stirring for 1/2 h under dry atmosphere, the alkoxides were hydrolyzed by distilled water addition. Precipitated raw powder was several times decanted by ethanol, dried at room temperature and then heated up 100 °C. Dry powder was divided to quarters and each of them was heated at temperature 800, 900, 1,000 or 1,100 °C respectively for 1 h in static air atmosphere.
2.2 Experimental technique
X-ray patterns were measured at room temperature on Siemens D5005 diffractometer with a Cu anode. A transmission electron microscope (TEM) was used for direct observation of the particles. Particle size determination was carried out using Scion Images software. The Mössbauer measurement was done in the transmission mode with 57Co diffused into a Cr matrix as the source moving with constant acceleration. The spectrometer was calibrated by means of a standard Fe foil and the isomer shift was expressed with respect to this standard at 300 K. Measurements were taken at 4 K with magnetic field 6 T. The fitting of the spectra was performed with help of a commercial program. The magnetization measurements were carried out in Quantum Design PPMS (Physical Property Measurement System) at 10, 100, 200, 300, and 350 K.
3 Results and discussion
3.1 X-ray diffraction measurement
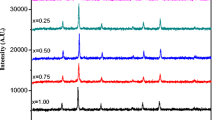
X-ray diffraction measurements were carried out on all samples. A relatively long measurements time was chosen (step 0.050° 2θ, time 45 s/step) and results are depicted in Fig. 1. The peaks position coincided with the characteristic peaks of the standard spinel phase. So, that the spinel structure was found as predominant phase in patterns for all annealing temperatures and negligible amount magnesium oxide was found in the samples but no other phases were detected. The diffraction patterns exhibit sharp peaks at all temperatures treatment which corresponds to well-crystallized solid phases. From the broadening effect of the peaks, the average particle size was determined about 31–38 nm (see Table 1).
3.2 TEM observation
The particle size observation by means of TEM confirms the tendency shown by the X-ray diffraction. The particle size increases only slightly with increasing heating temperature. From the Fig. 2 we can see that some particles are larger than 200 nm and some particles are smaller than 16 nm. The biggest particles are so large because the particles are aggregated into one big particle. And therefore the average particles size is about 39 nm (Table 1) from TEM. Figure 2 represents TEM micrography of the MgFe2O4 sample annealed at 800 °C. This figure was used to calculate the mean particle size.
3.3 Magnetic measurements
The magnetization of nanocrystalline MgFe2O4 was determined for sample prepared at 800 °C. Measurements were done at 10, 100, 200, 300, and 350 K. From Fig. 3, it could be seen, that saturation magnetization values increase with decreasing temperature of measure, and their values are 29, 32, 38, 44, and 47 Am2/kg at 350, 300, 200, 100, and 10 K respectively. Coercivities are zero at 350, 300, 200, and 100 K but we can see very small open hysteresis loop with a low coercivity at 10 K.
Figure 4 shows dependence of magnetization versus temperature. The lower curve is obtained for the sample cooled down to 10 K in zero field (ZFC), while the upper curve reflects the sample cooled down in a field of 10 mT (FC). In the maximum of the ZFC curve, which is observed at 350 K (T B) we can see the division of ZFC and FC magnetization curves. The results of the measurements ZFC and FC reveal ferrimagnetic character nanoparticles until 350 K (T B). Above this temperature ZFC and FC measurements reveal superparamagnetic character. This is probably due to the time of measurement and rotation of magnetic moment. The time of the magnetic measurement is longer than the relaxation time of the magnetic moment of nanoparticles, for this reason we can see the connection of ZFC and FC magnetization curves. When we compare the results from measurement of hysteresis and ZFC and FC measurements, we can say that the nanoparticles have ferrimagnetic character. The FC magnetization slowly increases with decreasing temperature until 20 K where the saturation is found FC. Magnetic measurement showed a ferrimagnetic character of all samples.
In the following section it were compared the magnetic properties of the prepared magnesium ferrite (by microemulsion method) with magnesium ferrite in silica matrix (by sol–gel method). Magnesium ferrite has zero coercivity at 350, 300, 200, and 100 K but at 10 K it has low coercivity. Magnesium ferrite in silica matrix has zero coercivity at 100 and 300 K but the hysteresis appears in magnetization curves at 2 K with larger coercivity than in magnesium ferrite. ZFC and FC measurement, demonstrated that MgFe2O4 nanoparticles have blocking temperature in the range of 340–360 K, whereas MgFe2O4/SiO2 nanocomposites have a blocking temperature about 10 K. It is clear that the samples prepared by sol–gel method and calcined at lower (800 °C) temperature are superparamgnetic and at higher (900, 1,000, 1,100 °C) temperatures have a ferrimagnetic character. While the samples prepared by microemulsion method treated at 800, 900, 1,000, and 1,100 °C have a ferrimagnetic character, which is caused by different size of particle.
3.4 Mössbauer spectroscopy
Mössbauer spectroscopy is a popular experimental technique for studying the microscopic magnetic properties of various ferrite systems. The X-ray diffraction did not give a satisfactory interpretation of our systems. For this reason, Mössbauer spectroscopy was used for the analysis.
Table 2 represents the data inferred from the measurements carried out at 4 K and 6 T. Figure 5 shows the spectra of the sample annealed at 1,100 °C. All samples measurement at 4 K and 6 T show two sextets belong to nanoparticles have a ferrimagnetic character. We can write to the formula MgFe2O4 as (Mg1−x Fe x )[Mg x Fe2−x ]O4, where x is obtained from the relative substitution of the iron in tetrahedral and octahedral position. The distribution of cations in tetrahedral and octahedral positions was inferred from Mössbauer data as follows: for the sample annealed at 900 °C as (Mg0.7Fe0.3)[Mg0.3Fe1.7]O4, for the sample annealed at 1,000 °C as (Mg0.68Fe0.32)[Mg0.37Fe1.68]O4 and for the sample annealed at 1,100 °C as (Mg0.64Fe0.36)[Mg0.36Fe1.64]O4. Differences in the distribution of cations are influenced by the temperature processing, these distribution of cations in tetrahedral and octahedral position affects the properties of the material but amount of cations remains the same. The amount of iron in tetrahedral position grow at the expense of the octahedral position with increasing temperature processing. The Mössbauer spectroscopy confirmed the presence of an ordered phase MgFe2O4 at 4 K and 6 T, where MgFe2O4 has a partial inversion of the spinel structure.
4 Conclusion
The metallic alkoxides in alcoholic solutions were used as ferrite precursors. The microemulsions used for next preparation consisted of cyclohexane, distilled water, 1-octanol and Lutensol ON 110.
The Mössbauer spectra and X-ray diffraction patterns confirmed the presence of MgFe2O4. The particle size of MgFe2O4 nanoparticles was calculated using Scherrer`s equation to 31–38 nm for samples heated form 800 to 1,100 °C. TEM showed the nanoparticles with the size about 39 nm. True the use of microemulsion method, it is possible to produce nanoparticles with narrow size distribution. Heat treatment temperature has only slight influence on the particle size in comparison with the influence of the microemulsion composition. The saturation magnetization values increase with decreasing temperature. ZFC-FC measurements, demonstrated that nanoparticles blocking temperature of the 800 °C sample was about 350 K. Hysteresis measurements and ZFC, FC curves of the MgFe2O4 nanoparticles revealed ferrimagnetic character at temperatures up to 350 K. The structural formula of MgFe2O4 is usually written as (Mg1−x Fe x )[Mg x Fe2−x ]O4, where round and square brackets denote sites of tetrahedral (A) and octahedral [B] coordination respectively, and where x represents the degree inversion. The degree of inversion, calculated from the subspectral area values. So that the formula can be written as (Mg0.7Fe0.3)[Mg0.3Fe1.7]O4 for the sample annealed at 900 °C, for the sample annealed at 1,000 °C as (Mg0.68Fe0.32)[Mg0.37Fe1.68]O4 and for the sample annealed at 1,100 °C as (Mg0.64Fe0.36)[Mg0.36Fe1.64]O4. Magnesium ferrite prepared via microemulsion method has a partial inversion of the spinel structure and therefore magnesium ferrite exhibit a ferrimagnetic behavior at low temperature.
The results show that nanocomposites prepared by sol–gel method contain smaller particles than nanoparticles prepared by microemulsion method. However the nanoparticles prepared by microemulsion method are pure MgFe2O4 spinel phase unlike the sol–gel method.
The main result of this work, is innovative and successful utilization of microemulsion method for preparation ferrites. The results show that MgFe2O4 nanoparticles can be synthesized by this microemulsion method.
References
Pithan C, Shiratori Y, Dornseiffer J, Haegel FH, Magrez A, Waser R (2005) Microemulsion mediated synthesis of nanocrystalline (K x Na1−x )NbO3 powders. J Cryst Growth 280:191–200. doi:10.1016/j.jcrysgro.2005.03.038
Shiratori Y, Magrez A, Pithan C (2005) Particle size effect on the crystal structure symmetry of K0.5Na0.5NbO3. J Eur Ceram Soc 25:2075–2079. doi:10.1016/j.jeurceramsoc.2005.03.012
Herrig H, Hempelmann R (1996) A colloidal approach to nanometre-sized mixed oxide ceramic powders. Mater Lett 27:287–292. doi:10.1016/0167-577X(96)00011-0
Okamura H, Kent Bowen H (1986) Preparation of alkoxides for the synthesis of ceramics. Ceram Int 12:161–171. doi:10.1016/0272-8842(86)90039-8
Acknowledgments
The authors thanks for financial support of the Grant Agency of the Academy of Sciences of the Czech Republic 106/07/0949.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holec, P., Plocek, J., Nižňanský, D. et al. Preparation of MgFe2O4 nanoparticles by microemulsion method and their characterization. J Sol-Gel Sci Technol 51, 301–305 (2009). https://doi.org/10.1007/s10971-009-1962-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-009-1962-x