Abstract
The measured model age is an important signature to constrain the production history of an unknown nuclear material. The aim of this work was to validate a rapid, robust quantification scheme for bulk uranium materials, amenable to multiple detection platforms. This work describes a combination of stacked columns, vacuum assisted separations, automation and a suite of analysis techniques to determine the ages of uranium materials and CRMs of known production history. The methodology allows for the determination of 234U/230Th and 235U/231Pa atom ratios via a novel approach, starting with a three resin column separation to allow high throughput and rapid turnaround. The materials analysed have concordant ages with known production histories, leading to the potential for expanding this work to additional chronometers, and the approach offers nuclear forensic practitioners an additional, advantageous separation methodology in the analysis of bulk uranium materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nuclear forensics laboratories provide analysis of nuclear materials to support law enforcement [1]. A review article by Mayer et al. [2] summarises recent developments and case studies of the methodology in nuclear forensic investigations. Developments in radio chronometry have yielded flexible methodologies applicable to a wide variety of samples [3]. The results generated are calculated model ages with an uncertainty budget derived from all aspects of the measurement.
The utility of quantified ages is that they confer a time window since last processing. Age dating relies on numerous assumptions, such as closed system behaviour and highly efficient purification (typically greater than 107 separation factors, SFs) of the parent/daughter of interest during last processing [4].
Recent advances include the certification of reference materials for purification date and the improved measurement of fundamental constants used in decay calculations to reduce calculation uncertainty [5, 6]. A large section of the decay chain is in principal amenable to ultra-trace analysis, which is a current area of interest [7]. The two chronometer pairs, involving the daughters thorium-230 and protactinium-231, were chosen as they have sufficiently high atomic ratios, quantities and half-lives to allow for highly accurate determinations to be accomplished from a wide range of material types, enrichments and ages.
Collaborative inter-comparison exercises have shown multiple chronometer analysis to be the standard approach to aid in the understanding of the processing history of a material [8]. Concordance/discordance of chronometer pairs can aid in the interpretation of the processing history of the material [9].
Solutions for analysis require quantification of the number of atoms of parent nuclide and purification and quantification of the trace daughter constituents from the bulk matrix and isobaric/spectral interferences to greater than required SFs. This ensures solutions are amenable to analytical techniques. This requires multiple purification steps and concentration of daughter isotopes. Precision is achieved using isotope dilution and certified reference standards [10, 11].
Certain uranium materials, for example a less than 5 year old depleted uranium sample, contain only small quantities of daughter isotopes. This is overcome in our work by pre-concentrating trace daughters from large quantities of material in glovebox containment. This allows for the final separations to be completed at lower hazard categories in a clean environment which reduces radiation exposure time and increases sample turnaround. Multiple analytical techniques are also required, as in certain cases radiometric techniques may confer a benefit over mass spectrometric analysis, such as for short lived daughter products or yield tracer radioisotopes. Certain techniques may also be able to produce results in shorter time frames, which may be beneficial in an investigation, all data being complementary to interpretation.
This project aimed to develop a rapid, flexible separation methodology for the 234U/230Th and 235U/231Pa chronometers, which is rapid, accurate and precise, reproducible, and amenable to multiple analytical techniques. The work aims to expand upon recent developments within the wider radiochemical separation area [12]. We improve the speed of analysis by the determination of both chronometers from a novel initial tandem separation and the application of readily available automation technologies. The aim was to use certified reference materials and matrix matched in-house control materials of known provenance to validate the technique.
Herein we report the development of a rapid method utilising TK400 resin for protactinium, AG1-X8 for uranium, and UTEVA for thorium purification, in tandem. If required for mass spectrometric analysis, a standard AG1-X8 separation for removal of uranium matrix and purification of trace metals can be used prior to the three resin separation.
The method also uses TK400 for the calibration of a 233Pa yield tracer which is required on a periodic basis as no long lived protactinium isotope is available for use. The extraction chromatography (EC) resins (UTEVA and TK400) retain thorium and protactinium respectively at the same acid molarity and are amenable to automation. In the case of TK400, it also has the benefit over the alternative silica gel and quartz wool methods which cannot be easily adapted to tandem separations and vacuum assisted flow [13]. A consistent rapid purification method is essential in maintaining a calibrated working protactinium tracer which has a limited useful lifetime of approximately 2 months. A new application of this resin to protactinium ultra-trace purification from uranium is reported.
The methodologies developed were adapted to vacuum assisted flow, and subsequently to an automated separation system commercially available from Elemental Scientific (ESI). The solutions separated by all techniques have removed isobaric/radiometric interferences. The matrix and concentration can be adjusted to the analytical technique available. In this work for example, we focused on alpha spectrometry (AS) and thermal ionisation mass spectrometry (TIMS).
Calibration of standards, system suitability tests, process blanks, backgrounds and multiple replicate samples all increase the quantity of analyses required. The advantages of vacuum assisted separation and this triple method are that they allow for analytical data to be available earlier in an investigation [14, 15].
Experimental
All chemicals applied in this study were of Optima grade (Fisher, Loughborough, UK), tracers and reference materials were provided from calibrated stocks within the Atomic Weapons Establishment (AWE). Uranium (U3O8) radio-chronometry and isotopic standard reference materials U005a, U630 and U930 from New Brunswick Laboratory (NBL) were used in this study. Tracers SRM 4342 (230Th), SRM 4328c (229Th), Isotope Products Laboratories (233U), and Amersham UK NGZ44 (237Np) were used for isotope dilution, yield tracers or for purification of 233Pa. The radionuclide concentrations were quantified by ID-TIMS and ID-AS. Ultra-high purity (UHP) deionized water (Fisher, Loughbrough, UK) was used throughout. All acid solutions were considered to have expired within 1 month of creation and ultrapure PFA bottles (Romil, Cambridge, UK) were used for all sample transfers and elution from columns. Extraction chromatography resins were purchased from Triskem© (Belgium) and washed with UHP reagents prior to use. All samples were taken in duplicate, spiked and un-spiked, with a combined process blank utilised. The flow rate for gravity columns was set at 0.5 mL min−1 for elution, and 1–5 mL min−1 for conditioning unless otherwise stated. Vacuum separations were undertaken with a 12-port vacuum box from Triskem©, with conditioning flow rates at > 1 mL min−1 and an ESI PrepFast™ auto-separation system was used to purify fractions for analysis, using optimised method files of the separation mapped above. Gravity columns were prepared from slurry in 0.1 cm diameter, 5 cm length columns, conditioned before use. Vacuum box columns were prepared from slurry in 10 mm diameter, 2 cm cartridges supplied by Triskem©. Samples for alpha spectrometry (Ortec alpha ensemble, with Alphavision 7.0 software, calibrated using certified mixed uranium/plutonium standards) were prepared by either sequential aliquot evaporation on a pre-cleaned steel planchette, or micro co-precipitated onto Triskem resolve filters using UHP Ce(III) and hydrofluoric acid. 233Pa was determined by gamma spectroscopy using an Ortec high purity germanium gamma spectrometer calibrated with certified mixed gamma standards. The samples had variable counting times with greater than 10,000 counts minimum taken for analysis. Determination of uranium isotope ratios by TIMS was achieved by loading 10 ng of uranium sample onto a zone refined, carburised rhenium single filament and running an automatic peak jumping method on a Thermo Triton to collect data. NBS U005a was used for mass bias correction. The assay of the control materials and certified reference materials was carried out using a modified Davis and Grey method on a Metrohm auto titration system alongside certified assay standards to give weight percent uranium to 0.10% standard error [16].
The separation scheme is outlined in bullet pointed order below:
233Pa Separation from 237Np for yield tracer preparation
-
1.
Evaporate an aliquot of 237Np tracer to near dryness in a Teflon beaker.
-
2.
Condition the tracer with 3 × 0.1 mL 11 M HCl.
-
3.
Re-dissolve the tracer in 1 mL 11 M HCl.
-
4.
Prepare a vacuum box cartridge or Rockbourne column containing 2 mL of TK400 resin.
-
5.
Condition the TK400 resin with 4 mL 11 M HCl. under vacuum.
-
6.
Place a Teflon beaker or Liquid Scintillation Counting (LSC), vial under the column and then load the tracer solution.
-
7.
Rinse and transfer with 3 × 0.2 mL 11 M HCl.
-
8.
Elute Np fraction with a further 10 mL 11 M HCl. under vacuum.
-
9.
Elute the Pa fraction into a new beaker/vial using 5 mL 5.5 M HCl/0.1 M HF.
-
10.
Evaporate the Np fraction to near dryness, and then re-dissolve in an appropriate mass of Optima grade 11 M HCl for re-use.
-
11.
Evaporate the Pa fraction to near dryness.
-
12.
Re-dissolve the fraction in 2 mL 11 M HCl and transfer to a pre-weighed LSC vial for counting by high resolution gamma spectroscopy.
Note The following methodology is also appropriate for individual chronometer separation of Pa/U.
Combined uranium/thorium/protactinium separation
-
1.
Add an appropriate quantity of 229Th and 233Pa tracer to the PFA vial/Teflon beaker containing the pre-concentrated U sample.
-
2.
Evaporate the sample to near dryness.
-
3.
Condition the sample with 3 × 1 mL 11 M HCl (evaporating to near dryness between additions).
-
4.
Re-dissolve the sample in 0.5 mL of 11 M HCl.
-
5.
Prepare three cartridge columns in the following order containing 2 mL respectively of TK400 resin, AG1-X8 (100–200 mesh) and UTEVA.
-
6.
Condition the columns with 6 mL 1 M HCl followed by 6 mL 11 M HCl.
-
7.
Transfer the sample volume onto the top of the column stack, and then rinse and transfer the washes from the sample beaker to the column with 3 × 0.3 mL 11 M HCl.
-
8.
Rinse the column stack with 5 mL 11 M HCl before separating into three individual columns for further purification.
To the TK400 column for Pa (attach another pre-conditioned TK400 column)
-
1.
Wash the column with a further 10 mL Optima grade 11 M HCl to elute U.
-
2.
Elute the Pa fraction into a suitable pre-weighed vial/beaker with 6 mL 5.5 M HCl/0.1 M HF.
-
3.
Prepare for either radiometric or mass spectrometric analysis.
To the AG1-X8 column for U (attach another pre-conditioned AG1-X8 column)
-
1.
Elute the U fraction with 6 mL 1 M HCl followed by 5 mL 0.5 M HCl and 5 mL 0.01 M HCl. Collect fractions from elution.
-
2.
Evaporate the U fraction to near dryness and/or prepare an aliquot for analysis.
To the UTEVA column for Th
-
1.
Wash the column with 5 mL 11 M HCl. (to waste).
-
2.
Elute Th fraction using 5 mL 5.5 M HCl; adjust to 11 M HCl.
-
3.
Prepare a pre-conditioned UTEVA column as outlined above (100–200 mesh).
-
4.
Condition the column with 5 mL 1 M HCl followed by 5 mL 11 M HCl.
-
5.
Load the sample onto the top column.
-
6.
Rinse the sample beaker with 1 mL 11 M HCl and add to the column.
-
7.
Wash dual column using 5 mL 11 M HCl.
-
8.
Elute Th using a further 5 mL 5.5 M HCl.
-
9.
Prepare for analysis by either alpha spectrometry or mass spectrometry.
Results and discussion
Separation chemistry for Pa and Th with a minimum 107 separation factor was desirable to ensure isobaric and radiometric interferences (Np/U) are removed. A 30 year old depleted uranium material of known provenance and NBS U630 were used for validation, covering oxide and metal matrices respectively.
Bulk solutions are prepared from up to one gram of sample, depending on material type and enrichment. Aliquots of solution containing at least 1010 atoms of both daughter isotopes (230Th and 231Pa) are sampled in duplicate for analysis.
If required, initial separation of uranium can be performed in a glovebox using AG1-X8 resin, when sample aliquots would not contain 1010 atoms of daughter isotopes. The sample is then transferred to a fume hood for further processing. This separation can also be employed if the sample contains large amounts of matrix elements, for example aluminum, which can interfere with resin capacity [17].
The methodologies in the literature were investigated and a tandem UTEVA separation was developed from the reported literature method for 230Th/234U [18]. This was then transferred to the vacuum box and the elution profile optimised. UTEVA showed the most reproducible performance over combined anion exchange/extraction chromatography methods reported [3]. In addition, the total combined type A and B uncertainty, U, in the developed method by alpha spectrometry was 3.6% in both cases and was unaffected in analysis of variance (ANOVA) on the data collected by separations under vacuum and gravity to test for flow rate effects. The average recoveries were 78 ± 3.6% from 30 replicate pairs, at this point it was decided that no additional development was required to quantify uncertainty due to the change in flow rates. The average separation factor per column was greater than 105 from a uranium sample with a wide range of potential interfering trace elements; allowing for complex matrix and sample types to be analysed, and give a solution for serial dilution and analysis.
Methods reported in the literature for purification of 231Pa/235U and 233Pa/237Np were identified [13, 14] and tested in this study. Silica gel (80 ± 2.7% recovery, SF 7 × 105), TK400 (91 ± 3.8% recovery, SF 6 × 104), quartz wool (52 ± 2.5% recovery, SF 4 × 104) and AG1-X8 (81 ± 1.6% recovery, SF 1 × 106) methods were tested for recovery and SFs of parent and daughter. Based on these results, larger resin beds or dual columns would be required to meet the required SFs.
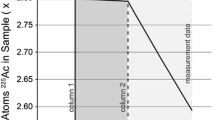
TK400 resin, which utilises the solvent 1-octanol on Amberchrome™, was investigated further. In hydrochloric acid, a ligand-chloride complex is formed preferentially with protactinium. At high chloride molarity, (> 9 M), neptunium, uranium, thorium, yttrium, barium and plutonium are weakly retained with SF < 10 in all cases allowing for a separation to be achieved [13]. The resin requires fewer chemical changes as adjusting acid molarity is sufficient to achieve a separation, without redox reagents. The method also requires fewer evaporation steps and reagents than the silica gel and anion exchange methodologies. Care must be taken to avoid PTFE column frits used in exchange chromatography columns, as these were shown to preferentially co-extract protactinium. In addition, hydrofluoric acid and hydrochloric acid concentrations should be accurate throughout, otherwise neptunium can co-extract into the protactinium elution and adsorption/hydrolysis can readily occur. Minimum elution volumes of 4.5 mL were determined to be sufficient for recovery of protactinium from a 2 mL packed column on the vacuum box and SF greater than 1010 (237Np/233Pa) were achieved from a dual TK400 column to calibrate the tracer. The separation scheme is outlined in Fig. 1 below:
The scheme was tested on replicates of U630 (Certified 06/06/1989 ± 190 days k = 2), yielding a model age of 07/04/1989 ± 106 days (k = 2) via alpha spectrometry for 230Th and TIMS for 234U from the date of analysis. A set of test samples were also spiked with 10 mg mL−1 of Al(III), Fe(II) and Si(IV), pre screened for uranium and protactinium, to which ages within the certificate value above were obtained. This added additional confidence for the wider applicability of the method, for example in the analysis of environmental samples for U/Pa/Th isotope fractions.
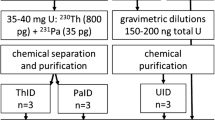
The two schemes were combined (Fig. 2) to give a separation that could afford both chronometer pairs and reduce the sample size required. The samples in this case containing ~ 1011 atoms of daughter nuclide is dissolved in 11 M HCl, homogenised with spike tracer and evaporated to 0.5 mL. The sample is loaded onto a triple column of TK400/AG1-X8/UTEVA conditioned in 11 M HCl. Alongside this, second stage columns of TK400/AG1-X8/UTEVA are conditioned with 11 M HCl on separate slots on the vacuum box. The initial triple column is washed with 5 mL 11 M HCl, then separated and each column is attached to an additional column of the same extractant in order to achieve the required separation factor. This is under vacuum assisted flow, allowing for the procedure to be completed in a few hours. The eluted fractions are then diluted or sub-sampled to give purified solutions suitable for radiometric and mass spectrometry techniques.
The results for the combined method were consistent with those observed from the methods separately, yielding certificate values for U630. The combined total dataset was used for validation. All analysis results from over 30 replicate pairs aged to a common reference date yielded uncertainties consistent with the uncertainty budget for each measurement technique.
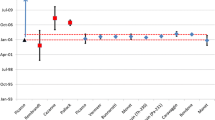
To test the method further, samples from the International Technical Working Group on Nuclear Forensics (ITWG) intercomparison exercise CMX-4 were analyzed. The two powders and one fuel pellet received were analysed alongside U630 and an in-house control material. The solutions were analysed using TIMS for uranium isotopes and Isotope Dilution-Alpha Spectrometry for Pa/Th atom ratios. The calculated ages were corrected to 03/10/2014 to align with the inital excercise results in Table 1 [19].
The results obtained agree with the consensus value reported by 11 laboratories in the excercise [18]. The enrichment date from the paper records of ES2 is 12.0 years from the reference date (Average Age: Feb–September 2002) and the average ages determined for ES1 and ES3 by all laboratories were 9.9 and 9.8 years respectively with uncertainties depending on the measurement (January–December 2004). Therefore the results agree with the approximate date of last purification. This showed the method to be suitable for operational use and further development. The chronometers pairs are concordant in each case and the ages of ES-2 and ES-1 and ES-3 could be used for discrimination, as the precision of the method is suitable to distinguish between the ages of the samples of interest and could be further improved with the use of more precise analytical techniques such as TIMS or MC-ICP-MS for Th and Pa determination. The method developed was also utilised in the recent ITWG CMX-5 intercomparison excercise; the materials were two 5 year old, < 1% enriched uranium fuel pellets, meaning the 231Pa/235U chronometer could not be employed, and were challenging for trace level age dating. In this case, the separation chemistry was completed and the reported result for the 230Th/234U was in agreement with the consensus value and in agreement with the twenty laboratories who submitted results [19].
The individual chronometer separation schemes discussed above were also developed for use on an ESI PrepFast autoseparator system, using the same elution volumes reported above. The system is a single loop and column with multi-port valves to add reagents. In the initial work reported here, it was possible to obtain similar separation factors using a 1 g column of TK400 and UTEVA for the U/Th (1 × 105) and Pa/U (1 × 104) respectively from simulant elemental standard solutions of 100 ppm U/Th and 1 kBq Pa/100 ppm U solutions.
Conclusions
A staged chemical separation method has been developed which yields high purity solutions of protactinium, uranium and thorium for quantification from a novel triple column approach. The method demonstrates a wider application of the TK400 resin in the purification of protactinium from uranium and trace elements to aid in a nuclear forensic investigation.
A rapid separation of the two most commonly applied uranium chronometer pairs from uranium materials of varying types and enrichments to specified SFs was targeted. Certified Reference Materials and inter-comparison samples of known provenance were used to validate the procedure. The chronometers were concordant in the cases analyzed and the results were within certified/consensus values, demonstrating the robustness of the method to sample type. Further work on discordant chronometer pairs, such as those demonstrated in the CMX-3 exercise would be a useful additional investigation [20].
The method has additional advantages over those published in the literature currently. One advantage is the fast turnaround time by utilizing vacuum assisted flow and/or automation. The scheme is flexible as additional purification columns can be utilized depending on the application should additional actinides or trace element separations be required. The method also avoids the use of glass wool/silica gel which can be laborious, uses smaller volumes of reagents and has less evaporation stages that other methods in the literature. The columns can be also re-used for periodic purification of the 233Pa tracer.
The study also involved analysis with additional background elements added for robustness testing which in principal allows for the methods to be more widely applied in environmental radiochemistry where rapid methods are advantageous for routine or emergency samples. The method was also tested in international inter-comparison exercises, reporting the consensus value in both cases. Future work will look to expand the methodologies to the wider decay chain should granddaughter chronometers require analysis, and apply the automation to a wider range of materials to enhance capability.
References
Mayer K, Wallenius M, Ray I (2005) Nuclear forensics—a methodology providing clues on the origin of illicitly trafficked nuclear materials. Analyst 130:433–441
Mayer K, Wallenius M, Varga Z (2012) Correlating measurable material parameters to the history of nuclear material. Chem Rev 113:884–900
Varga Z, Mayer K, Bonamici CE, Hubert A, Hutcheon I, Kinman W, Kristo M, Pointurier F, Spenser K, Stanley F, Steiner R, Tandon L, Williams R (2015) Validation of reference materials for uranium radiochronometry in the frame of nuclear forensic investigations. Appl Radiat Isot 103:81–86. https://doi.org/10.1016/j.apradiso.2015.05.005
Sturm M, Richter S, Aregbe Y, Wellum R, Mialle S, Mayer K, Prohaska T (2014) Evaluation of chronometers in plutonium age determination for nuclear forensics: what if the ‘Pu/U clocks’ do not match? J Radioanal Nucl Chem 302:399–411
Varga Z, Venchiarutti C, Nicholl A, Krajko J, Jakobic R, Mayer K, Richter S, Aregbe Y (2015) IRMM-1000a and IRMM-1000b uranium reference materials certified for the production date. Part I: methodology, preparation and target characteristics. J Radioannal Nucl Chem 307:1077–1085. https://doi.org/10.1007/s10967-015-4227-x
Varga Z, Nicholl A, Wallenius M, Mayer K (2016) Re-measurement of (234)U half-life. Anal Chem 88:2763–2769. https://doi.org/10.1021/acs.analchem.5b04370
Rolison JM, Treinen KC, McHugh KC et al (2017) J Radioanal Nucl Chem 314:2459. https://doi.org/10.1007/s10967-017-5619-x
Nuclear Forensics International Technical Working Group (ITWG) Round Robin 3 Exercise after action and lessons learned report. Coordinator: Hanlan R (2010) Pacific Northwest National Laboratory, pp 78
Varga Z, Nicholl A, Wallenius M, Mayer K (2012) Development and validation of a methodology for uranium radiochronometry reference material preparation. Anal Chim Acta 718:25–31
LaMont SP, Hall G (2005) Uranium age determination by measuring the 230Th/234U ratio. J Radioanal Nucl Chem 264:423–427
Keegan RP, Gehrke RJ (2003) A method to determine the time since last purification of weapons grade plutonium. Appl Radiat Isot 59:137–143
Jerome S, Collins S, Happel S, Ivanov P, Russell B (2017) Isolation and purification of protactinium-231. Appl Radiat Isot. https://doi.org/10.1016/j.apradiso.2017.07.051
Morgenstern A, Apostolidis C, Mayer Klaus (2002) Age determination of highly enriched uranium: separation and analysis of 231Pa. Anal Chem 74:5513–5516. https://doi.org/10.1021/ac0203948
Wallenius M, Mayer K, Ray I (2006) Nuclear forensic investigations: two case studies. For Sci Int 156:55–62. https://doi.org/10.1016/j.forsciint.2004.12.029
Maxwell SL, Culligan BK, Hutchison J (2014) Rapid determination of actinides in asphalt samples. J Radioanal Nucl Chem 299:1891–1901. https://doi.org/10.1007/s10967-013-2885-0
Davies W, Gray W (1964) A rapid and specific titrimetric method for the precise determination of uranium using iron (II) sulphate as reductant. Talanta 11:1203–1211
Kayzar TM, Williams RW (2016) Developing 226Ra and 227Ac age-dating techniques for nuclear forensics to gain insight from concordant and non-concordant radiochronometers. J Radioanal Nucl Chem 302:2061–2068
Rolison J, Treinen C, McHugh K, Gaffney A, Williams R (2017) Application of the 226Ra–230Th–234U and 227Ac–231Pa–235U radiochronometers to uranium certified reference materials. J Radioanal Nucl Chem 314:2459–2467. https://doi.org/10.1007/s10967-017-5619-x
Nuclear Forensics International Technical Working Group (ITWG) Collaborative materials exchange exercise after action and lessons learned report 4. Coordinator: Schwantes J (29/10/2015) Pacific Northwest National Laboratory. PNNL-24410
Nuclear Forensics International Technical Working Group (ITWG) Round Robin 3 Exercise after action and lessons learned report 3. Coordinator: Hanlen R (25/04/2011), Pacific Northwest National Laboratory. PNNL-20079
Acknowledgements
The research herein was funded and supported by AWE plc, UK. Paul Thompson, James Dunne and Nathan Thomas are thanked for help guiding this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Higginson, M., Gilligan, C., Taylor, F. et al. Development of rapid methodologies for uranium age dating. J Radioanal Nucl Chem 318, 157–164 (2018). https://doi.org/10.1007/s10967-018-6021-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-6021-z