Abstract
Environmental isotopic study has been conducted in Faridkot and Muktsar districts of Punjab to assess the uranium contamination as well as to determine the isotopic signatures of contaminated groundwater. Results indicate that U levels range from 3 to 190 µg/L and ten samples are contaminated out of twenty collected. Uranium contamination is observed both in shallow and deep groundwaters but decreases with depth. Positive correlation was found between alkalinity and U. The δ18O values range between −9 and −6‰ and do not show any distinction between contaminated and uncontaminated groundwater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uranium is a naturally occurring, radioactive element present in certain types of rocks and soils. It occurs in dispersed state and has a heterogeneous distribution in the earth due to geochemical processes. High concentration of uranium in drinking water and foodstuffs has potential harmful effects on human beings because of its chemical toxicity. The biokinetics, metabolism and chemical toxicity of uranium including its effect on kidney function is well established [1]. Chemically, uranium has nephrotoxic effect i.e. it affects the kidney of organism when ingested [2]. The specific activity of uranium being low i.e. 25 mBq/L it has less radiotoxic effect on body compared to its chemical toxicity. There are many reports on health issues related to U contamination [3, 4]. Mobilization of uranium depends on the pH, which changes the oxidation state and speciation of metal ion, which in turn controls the solubility in groundwater. It has been studied that uranium solubility increases with increase in bicarbonate concentration because of carbonate complexation [5]. Uranium exists mainly in U4+ and U6+ states. In anoxic condition U(IV) forms insoluble complexes and precipitates while in oxic condition it oxidizes to U(VI) and forms soluble complex and moves in the groundwater system. U(IV) dominates at Eh < 200 mV for water logged/wet soils whereas U(VI) predominates in sufficiently aerated soil [6]. The groundwater and host rock minerals interactes, thus both chemical and physical differentiation processes are enhanced according to rock-water ratio, surface area exposed and residence time [7]. Studies on uranium contamination in Punjab region was undertaken by many agencies but were mainly limited to profiling in water and soil. Earlier studies indicate that the uranium occurrences have been encountered not only at the shallow depth but also in deeper depths. Different hypothesis have been proposed to understand the U dissolution in water based on several studies in India [8] and abroad [9], however there are still some unresolved complexities that needs to be addressed in order to clearly ascribe the causes behind the widespread uranium contamination in groundwater of southwestern Punjab.
Stable isotopes of H (δ2H) and O (δ18O) are widely employed to understand groundwater recharge and its interactions with surface water or precipitation [10]. The application of stable isotopes to study recharge is based on the spatial and temporal variation of the isotopes of elements of water molecules. During evaporation heavier isotopes concentrate in the liquid phase while lighter isotopes concentrate in the vapor phase. This is termed as fractionation, which occurs due to differences in diffusion velocities and zero point energies [11]. The isotopic compostion of precipitaion is also affected by season, latitude, altitude, amount and distance from the coast [12]. Based on the fractionation and other variations, the stable isotope of groundwater is compared with stable isotope of surface water, precipitation water of the study area and a correlation is established by plotting stable isotope along with the Global Meteoric Water Line (GMWL, δ2H = 8 × δ18O+10). GMWL is locus of precipitation water over whole of the globe [13] and used to describe the environment of water. Any deviation from GMWL indicates processes like evaporation, condensation, geothermal and microbial activity etc. The standard used for measurment of stable isotope is Vienna Standard Mean Oceanic Water provided by IAEA, Vienna. The delta values assigned for δ18O and δ2H for VSMOW are 0‰. The study area is covered extensively by the canal network of Sirhind feeder canal to meet the irrigation and drinking water needs of the people. The climate of the district is dry sub humid with grass land type of vegetation and receives an annual rainfall of 431 mm in 22 rainy days. 79% of the annual rainfall occurs during monsoon period and 21% occurs during nonmonsoon period. Beside uranium contamination almost whole of the district is facing water logging problem majorly affecting southern and north western parts of district [14]. Due to water logging issue the fertile land is becoming unfit for agriculture. The groundwater is highly saline with electrical conductivity (EC) ranging between 1000 and 6900 µS/cm. The maximum uranium concentration in Faridkot district was found to be 476 µg/L with average value of 68 µg/L [15]. This study is an attempt to understand the geochemical controls on uranium distribution in groundwater of Muktsar and Faridkot districts and to study the variation of stable isotope in groundwater of this region.
Study area
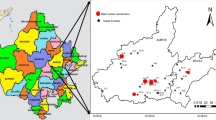
Muktsar and Faridkot districts covered in this study fall in the southwest part of Punjab state. Study area covers region between 29°53′ and 30°49′ north latitudes and 74°14′ and 75°3′ east longitudes, and consists of Quaternary alluvial deposits. The study area is bounded by Bathinda district in the east, Fazilka district in west, Moga and Ferozpur districts in north and Rajasthan in South. The sediments of the alluvial plains are of Recent to Sub-Recent age and can be classified into two unit i.e. newer alluvium and older alluvium. The newer alluvium is generally confined to the river channels and is laid by flood water whereas older alluvium consists of pale to reddish brown colored layers of massive clay. These alluvial deposits were formed by the sediments transported from adjoining areas comprising Siwaliks, granites and other metamorphic rocks [16]. The study area is mainly dependent on the groundwater to meet the demands for agriculture and drinking purposes. Intense irrigation practices have led to depletion of groundwater near to 1 m/year in major parts of the Punjab state [17]. The decline at Muktsar (2002–2011) is 0.031 m/year whereas at Bhaliana, decline is 0.21 m/year (2002–2011) which is a nominal decline in 10 years (2002–2011). All the other wells show rise in water levels ranging from 0.008 (Labianwali) to 0.322 m/year (Kuthian wali) [14].
Experimental
A total of 20 samples were collected from bore wells, hand pumps in the study area (Fig. 1). Physical parameters like, temperature, pH, dissolved oxygen, Total Dissolved Solids (TDS) and Electrical Conductivity (EC) were measured in situ. Alkalinity was determined in the field using Gran titration method. Water samples were filtered using 0.45 µm filters and stored in acid treated sample bottles. Uranium measurement was done by fluorimeter (UA1, Quantalase), which works on the principle of fluorescence of uranyl complex enhanced by addition of sodium pyrophosphate as fluorescence enhancement reagent [18]. The detection limit was determined by standard addition method, in which 5–20 µg/L of standard uranium solution was spiked into the water sample in increments of 5 µg/L and the background signal (noise) due to sample matrix and instrument were measured from the y-intercept of the best fit equation. The typical detection limit is 0.2 µg/L. A suitable dilution was done in the case of sample containing high uranium content. The validation for U measurements was carried out using another established analytical method, in this case ICP-MS. The results of which are published by Rishi et al. [19]. Environmental isotopes (δ2H, δ18O) are analyzed by isotope ratio mass spectrometer (IsoPrime 100) using pyrolysis mode of elemental analyzer for deuterium and gas equilibration method for δ 18O [20]. The precisions (2 sigma) of δ2H and δ18O are ±0.15 and ±0.1‰ respectively.
Results and discussion
Uranium concentrations in the water samples were found in the range of 3–190 µg/L for Muktsar with an average of 43 and 4–171 µg/L for Faridkot with an average of 67 µg/L. Majority of the groundwater was found to contain uranium concentration above drinking water permissible limit of 30 µg/L [21, 22]. In Muktsar 44.4% samples are contaminated while in faridkot 81.8% samples are contaminated. The pie charts of the level of contamination is shown in Fig. 2a, b. The groundwater is contaminated by U in the adjoining districts such as Mansa and Bathinda as well. The U concentration ranges from 29 to 621 μg/L in Mansa district and 22.2–352 μg/L in Bathinda district [19]. Uranium contamination is more prominent in Faridkot district than in Muktsar district.
From depth profile of uranium (Fig. 3) it is seen that few shallow and deep zones are uranium contaminated. It is observed that up to 30 m depth the uranium contamination is high. Decreasing contamination trend is observed with increasing depth. The main reason for decrease in U along the depth can be attributed to bicarbonate concentration. The deep zone also shows a reduced amount of bicarbonate concentration. Similar results were reported by Rishi et al. [19].
δ18O values for groundwater of Muktsar and Faridkot ranges from −10.16 to −5.93‰ and −9.15 to −2.82‰ respectively, while δ2H values are −63.7 to −36.2‰ and −60.3 to −31.9‰ respectively. The stable isotope plot (Fig. 4) indicates that groundwater is recharged by precipitation water as the samples fall on GMWL.
The depth profile of δ18O (Fig. 5) shows that there is not much difference between shallow and deep wells isotopically. The wells seems to be not affected by the inputs from return flow as there is no evaporation signature in the stable isotope values. The samples which undergo evaporation normally fall on a regression line with a slope <5. All the samples fall on the GMWL (except one) and the slope of the best fit line is 6.35 (Fig. 4). Therefore, it can be stated that there is no evaporation signature found in the water samples. Hence irrigation return flow might not be contributing to groundwater recharge at these locations. The enriched stable isotopic value of one sample indicates that the sample is receiving some recharge contribution from either evaporated water body present in the study area or contribution from irrigation return flow. The depleted stable isotopic value is an indication of contribution of higher altitude recharge source such as canal water.
Uranium contaminated groundwater are not distinguishable on the basis of its isotopic content as seen from δ18O versus uranium plot (Fig. 6). The isotope range for uranium contaminated samples is −9 to −6‰.
Figure 7 shows a good positive correlation between alkalinity and U up to 75 μg/L with r 2 = 0.61.The best fit line includes samples from 0 to 75 μg/L U excepting one sample with very high alkalinity (about 1250 mg/L). Higher values of U don’t show a proportional increase in alkalinity of the groundwater. The deeper wells show moderate alkalinity and U concentration and have higher concentration of chloride indicating evaporite dissolution [23].
Conclusions
An environmental isotope study has been conducted in southwest part of Punjab state, viz., Muktsar and Faridkot districts, to assess the uranium contamination in groundwater as well as to evalute the stable isotopic characteristics. The results indicate that uranium levels reach up to 190 µg/L. 44.4 and 81.8% of samples are contaminated with high uranium in Muktsar and Faridkot districts respectively. The uranium contamination is observed in both shallow and deep groundwaters, however deeper zones are relatively less contaminated. Environmental isotope study infers that isotopically there is no distinction between high U and low U groundwaters. U contamination resulting from anthropogenic activities (irrigation return flow) seems to be unlikely and the possible source of U could be geogenic.
References
Domingo JL (1995) Prevention by chelating agents of metal-induced development toxicity. Reprod Toxicol 9:105–113
Zamora ML, Tracy BL, Zielinski JM, Meyerhof DP, Moss MA (1998) Chronic ingestion of uranium in drinking water: a study of kidney bioeffects in humans. Toxicol Sci 43:68–77
Kurttio P, Auvinen A, Salonen L, Saha H, Pekkanen J, Mäkeläinen I, Väisänen SB, Penttilä IM, Komulainen H (2002) Renal effects of uranium in drinking water. Environ Health Perspect 110:4
Uranium in drinking-water, WHO/SDE/WSH/03.04/118/Rev/1
Echevarria G, Sheppard MI, Morel JL (2001) Effect of pH on the sorption of uranium in soils. J Environ Radioact 53(2):257–264
Abdelouas A, Lutze W, Nuttall E (1998) Chemical reactions of uranium in groundwater at a mill tailings site. J Contam Hydrol 34(4):343–361
Chkir N, Guendouz A, Zouari K, Ammar FH, Moulla AS (2009) Uranium isotopes in groundwater from the continental intercalaire aquifer in algerian tunisian sahara (North Africa). J Environ Radioact 100:649–656
Ajay Kumar, Tripathi RM, Rout S, Mishra MK, Ravi PM, Ghosh AK (2014) Characterization of groundwater composition In Punjab state with special emphasis on uranium content, speciation and mobility. Radiochim Acta 102(3):239–254
Tricca A, Porcelli D, Wasserburg GJ (2000) Factors controlling the groundwater transport of U, Th, Ra and Rn. J Earth Syst Sci 109(1):95–108
Negrel P, Petelet-Giraud E, Barbier J, Gautier E (2003) J Hydrol 277:248–267
Dansgaard W (1964) Stable isotope in precipitation. Tellus 4:436–468
Rozanski K, Araguas Araguas L, Gonfiantini R (1993) Isotopic pattern in modern global precipitation. In: Continental isotope indicators of climate. American Geophysical Union Monograph, vol 78 American Geophysical Union, Washington, DC, pp 1–36
Craig M (1961) Isotopic variations in meteoric waters. Science 133:1702–1703
Behera SC, Bhaita AK Muktsar, Punjab CGWB report. www.cgwb.gov.in/District_Profile/Punjab/Muktsar.pdf
Bajwa BS, Kumar S, Singh S, Sahoo SK, Tripathi RM (2016) Uranium and other heavy toxic elements distribution in the drinking water samples of SW-Punjab, India. J Radiat Res Appl Sci. http://dx.doi.org/10.1016/j.jrras.2015.01.002
Wadia DN (1981) Geology of India. Tata McGraw-Hill, New Delhi, p 508
Gupta S Regional Director groundwater management in Alluvial Areas Central ground water board. http://hindi.indiawaterportal.org/node/53383
Sahoo SK, Mohapatra S, Chakrabarty A, Sumesh CG, Jha VN, Tripathi RM, Puranik VD (2010) Determination of uranium at ultra-trace level in packed drinking water by laser fluorimeter and consequent ingestion dose. Radioprotection 45:55–66
Rishi MS, Tirumalesh K, Sharma DA, Pant D, Sinha UK (2017) Spatial trends in uranium distribution in groundwaters of Southwest Punjab, India—A hydrochemical perspective. J Radioanal Nucl Chem. doi:10.1007/s10967-017-5178-1
Lutz A, Thomas JM, Panorska A (2011) Environmental controls on stable isotope precipitation values over Mali and Niger, West Africa. Environ Earth Sci 62:1749–1759
US EPA (1999b) (United states Environmental Protection Agency): National primary drinking water regulation; radon-222. Proposed rule, Washington, DC
WHO (world health Organization): Guidelines for drinking water quality Uranium in drinking water (2012)
CGWB (2014) Water quality issues and challenges in Punjab. A report 160. http://cgwb.gov.in/NEW/WQ/Punjab%20Book%20Final%20for%20Printing.pdf
Acknowledgements
Authors sincerely acknowledge the constant support and encouragement by Mr. K.S.S.Sarma, Head, Isotope and Radiation Application Division and Dr. B.S. Tomar, Director, Radiochemistry and Isotope Group, Bhabha Atomic Research Centre, Mumbai. The authors would like to acknowledge the BRNS, DAE for providing the financial support (Letter No. 35/14/11/2014-BRNS-193).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pant, D., Keesari, T., Sharma, D. et al. Study on uranium contamination in groundwater of Faridkot and Muktsar districts of Punjab using stable isotopes of water. J Radioanal Nucl Chem 313, 635–639 (2017). https://doi.org/10.1007/s10967-017-5284-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5284-0