Abstract
In this paper the suitability of two non-destructive analytical techniques for identification of elemental signatures in samples containing uranium of different enrichments was studied. The measurements were based on particle induced X-ray emission (PIXE) and particle induced gamma-ray emission (PIGE) methods. The samples were irradiated by 3 and 5 MeV protons at the 3 MV Tandetron™ of Horia Hulubei National Institute for Physics and Nuclear Engineering. The characteristic X and gamma rays were measured using high purity germanium (HPGe) detectors. The GUPIX software was applied for processing the PIXE spectra, while a relative standardization was applied for PIGE analysis by using certified comparator standards and proton stopping powers calculated by SRIM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The European Union (EU) non-proliferation policy prioritizes the prevention of illicit trafficking and unauthorized possession of radioactive materials [1, 2]. Nuclear material seized in illicit trafficking may originate from several sources. Nuclear forensic science allows, by comparing signatures (chemical, physical, isotopic and elemental characteristics) with reference data from national nuclear forensic libraries, to determine the origin, the intended use, the last legal owner and the smuggling route of the analyzed sample. This information is essential, not only for the case investigation, but also for preventing future thefts and proliferation of radioactive materials [1–6]. One important fingerprint is represented by the metallic impurities present in uranium samples at various concentrations [7–17]. During the nuclear material purification process, the accompanying metallic impurities may be reduced by a factor of 103. Further, certain impurities diffuse into the material due to its contact with different media like container or storage tank. This process is not yet well known [2], however, it is known that the amount and type of minor elements are signatures of the investigated nuclear material which can be linked to the place of its production [7].
Sometimes, the material of interest for nuclear forensics is itself evidence in court. Therefore, non-destructive techniques of analysis are preferred. The characterization of nuclear material by atomic and nuclear techniques, using charged particles accelerators of the Horia Hulubei National Institute for Physics and Nuclear Engineering (IFIN-HH), can help identify the origin of the studied samples by determining their elemental composition, thus indicating the technologies used for their preparation, processing and the specific phase of the nuclear fuel cycle. Particle induced X-ray emission (PIXE) and particle induced gamma-ray emission (PIGE) techniques have been frequently employed for the search of trace elements in many types of matrices [18, 19] but very few analytical results have been found dealing with UO2 samples. This paper explores the suitability of PIXE and PIGE techniques as nondestructive systematic tools for nuclear forensic analysis and establishes some experimental conditions for a successful result. The main inorganic impurities in uranium samples from the nuclear industry are Al, Si, P, S, Cl, K, Ca, Cr, Mn, Fe, Ni, Cu, Zn, Zr, Mo and Pb [7–17]. Their mass fractions are of the order of mg kg−1 (ppm, parts per million) to percent. The analysis of minor and trace elements in uranium samples are generally performed by mass spectrometry techniques [7–12]. Nevertheless, atomic absorption/emission spectrometry [13], ion chromatography [14], energy dispersive X-ray fluorescence [15], neutron activation analysis [16], or ion beam activation analysis [17] could also be applied for this purpose.
Experimental
Uranium dioxide samples
Five uranium dioxide (UO2) samples available in the facility holding nuclear material of IFIN-HH were studied using ion beam analysis (IBA) at 3 MV Tandetron™ of the same institute [20]. The samples were of different enrichment (weight percent of 235U) levels. One was depleted, two natural, one highly enriched and one weapons grade uranium sample. The mass of the studied samples varied from 0.8 to 2.4 g (Table 1).
All the analyzed samples were wrapped into mylar foil (2.5 μm thickness) to avoid a possible radioactive contamination, and then placed on the sample holder inside the IBA reaction chamber. This foil produced a negligible modification of proton beam energy on the target (39 keV for 3 MeV protons and 26 keV for 5 MeV protons), as calculated by SRIM code [21]. The samples were individually measured to avoid reciprocal spectral interferences due to uranium self-induced X-rays and uranium decay chain gamma-rays.
IBA experimental setup
For PIXE, a 3 MeV collimated proton beam was used to irradiate the samples. The beam current was ranging from 1 to 10 nA for different samples. The electric charge (Q) varied from 8 to 13 µC. It was measured in the isolated sample holder connected to an electric charge integrator 20]. The targets were positioned perpendicular with respect to the beam direction.
The following detectors attached to the IBA vacuum chamber were used simultaneously to collect the spectra:
-
For PIXE (X-ray detection)-IGLET-X-06135-S HPGe (diameter 6 mm, depth 6 mm) with Be window of 0.0127 mm, inserted in the IBA vacuum chamber;
-
For PIGE (γ-ray detection)-GEM10P4-70 retractable HPGe gamma-ray detector (10% relative efficiency and energy resolution of 1.75 keV at 1.33 MeV of 60Co), situated at about 15 cm to the target (the closest position allowed by the IBA chamber of 50 cm diameter).
Both detectors were placed at 45° with respect to the beam direction [20, 22].
PIXE measurements were performed using a 40 μm Al filter to reduce the counting rate in the low energy region of the spectra and thus to improve the analytical sensitivity for higher Z elements (lower pile-up effects) [23]. The target to detector distance was of 12 cm (the closest position).
PIGE measurements were performed using 3 MeV proton beam energy for all the investigated samples and 5 MeV proton beam energy for two of the samples.
Rutherford backscattering spectrometry (RBS) technique was in addition used to test the correctness of the proton beam electrical charge value stated by IBA experimental set-up [20, 24]. Spectra obtained by the three techniques (RBS, PIXE and PIGE) were collected simultaneously.
Results and discussion
PIXE analysis
The obtained X-ray spectra were normalized to the electric charge integrated on the target, taking into account the dead time of the system.
In order to check the quality of the PIXE results, the data analyses were performed in parallel using different fitting procedures in two independent laboratories, obtaining similar results within the uncertainties: Laboratory GammaSpec of IFIN-HH, situated in Magurele, Romania, and Centro National de Aceleradores (CNA), situated in Sevilla, Spain [25].
NIST 1412 (multicomponent glass), covered with 100 nm graphite layer, was used as reference standard for PIXE calibration and data processing at the CNA institute. Due to the presence of the Mylar wrapping foil of 2.5 μm thickness used to avoid the contamination, the energy lost by the protons passing through it (39 keV) was taken into account for the fitting procedure. It was also added in the detector filters list, although in this case, its effect is negligible with respect to the effect of the 40 µm Al filter. The spectra were processed by GUPIX software [26] employing the Fixed Matrix Solution with UO2 as matrix. Aluminum was included in the fit element list as a parasitic element. Solid angle of the detector was calculated using the mentioned reference standard as the average value for the different X-ray energies [H = 0.002624, 5% standard deviation (SD)]. Hence, only one reference standard was needed. Detector efficiency was calculated by the GUPIX code using the “gucsa” subroutine and the nominal parameters of the detector.
At IFIN-HH, GUPIX program was used to run as Iterated Matrix Solution with oxygen as invisible element tied to visible elements. The calibration factors (H) were introduced as energy dependent, with an average value of 0.002603 ± 10% (SD) (in agreement with CNA value). They were determined based on IRMM metallic foils (Ti, Fe, Ni, Cu, and Nb) of certified mass fractions, measured in the same experimental configuration with the investigated samples.
A total of eight elements (impurities) were detected and quantified using PIXE method. Results obtained with CNA fitting procedure are shown in Table 2. The uncertainties provided in the table correspond to both statistical and fitting uncertainties, with coverage factor k = 1. Other sources of uncertainty are difficult to estimate. They are mostly part of the acquisition procedure. In fact, we normalized the proton charge to the uranium peak in order to avoid a charge measurement uncertainty found in the acquired spectra. The total uncertainty due to other acquisition systematic uncertainties by PIXE could be estimated at 5%.
To be noted, PIXE spectra were difficult to interpret due to uranium X-ray peaks, as well as Ge escape and sum peaks which overlapped the peaks of interest making the limits of detection (LOD) values for most of the interesting elements relatively high. As an example, PIXE spectra corresponding to the highly enriched uranium (HEU) sample (P5) measured without X-ray filter and with 40 μm Al filter at the maximum (26.9 cm) and minimum (12 cm) distance to detector, respectively, are shown in Fig. 1.
For a future analysis of this type of samples it seems important to use a silicon detector in order to avoid the more intense and numerous germanium escape peaks. In Fig. 2, the contribution of sum peaks and Ge escape peaks produced just by uranium L-X ray lines according to GUPIX estimation are shown.
The following LOD values (in mg kg−1) were found for the elements determined by PIXE (3 MeV protons) in the investigated samples: 20–30 (Zn, Ga, and Cu), 100–120 (Ni), 140–170 (Cr), 190–210 (Fe), 450–500 (Zr), and 700 (Ti).
Figure 3 comparatively presents the PIXE results obtained in both laboratories. As could be seen in this figure, the results were in a good agreement.
PIGE analysis
For each sample, 3 h uranium spectra were collected prior to the in-beam measurements, in the same geometrical set-up. This was done to distinguish between the beam particles induced prompt gamma rays and gamma rays coming from the decay of uranium isotopes and their daughter products. The total collected electrical charge for 5 MeV proton beam experiments was around 70 μC (irradiation time of about 3 h).
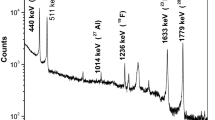
Figure 4 presents a PIGE spectrum for the P5 sample (93% enrichment) with observed nuclear reactions (upper spectrum of higher counting statistics), the corresponding uranium spectrum collected without a proton beam on target (lower spectrum), and the environmental background spectrum. All nuclear reactions which were used for PIGE analysis of the investigated UO2 samples are mentioned in Fig. 4. Nuclear reactions produced by the proton beam on oxygen isotopes present in the targed could also be seen in the spectra. The rest of the gamma peaks correspond to uranium isotopes, their daughter products and the natural background [27]. The gamma spectra were processed using the GammaW program [28].
For PIGE standardization, a relative analytical method was applied with standards of certified element concentration (mass fraction), using the following formula [23]:
where C sample and C standard, are the element concentrations in sample and standard, respectively; Y sample and Y standard, are element gamma-ray yields (net peak areas) for sample and standard, respectively, normalized to the beam charge of the incident protons (corrected by counting dead time); S sample and S standard, are stopping powers for proton beam of energy E 1/2.
The stopping power values for the uranium dioxide samples, as well as for chemical compounds used as comparator standards were determined using SRIM simulation program.
The proton beam energy E 1/2 is defined as Y(E 1/2) = Y(E p)/2, with E p as incident proton beam energy. To determine E1/2, excitation functions were measured for all standards using proton beam energies between 2.4 and 3 MeV (energy step of 0.1 MeV). A quantitative standardization for PIGE was performed using high purity metallic foils for Al and Ti (IRMM), stainless steel (SS-AISI316, Goodfellow) for Cr, Si monocrystalline wafer, and pellets of high purity chemical compounds (NaCl, Fe2P, CaF2, LiNH2, and MgO). In the case of UO2 samples, a stopping power value corresponding to 3 MeV proton beam energy was considered (systematic uncertainty lower than 5%).
Table 3 presents the results obtained by PIGE at IFIN-HH. Uncertainties in the table are calculated as combined counting uncertainty for sample and standard, as well as mass fraction uncertainty for standard (coverage factor k = 1). A systematic uncertainty due to stopping power corrections is assessed to be lower than 10%. The detection limits are corresponding to 3σ.
The number of minor elements excited by 3 MeV protons was rather low, therefore the proton beam energy was increased up to 5 MeV, and a larger variety of reactions were observed in the case of two samples (P3 and P5).
The quantitative standardization for PIGE at 5 MeV proton beam was not realized, it remains a question for further research. Nevertheless, the relative contents of minor and trace elements determined in the P3 and P5 samples (in counts/μC) are presented in Table 4. In the same table, the relative contents for some elements were compared with those obtained by different approaches (PIGE-3 MeV and PIXE CNA fitting procedure). In the P3 and P5 samples, the elements visible by PIGE both at 3 and 5 MeV proton beam were F, Na, Al and P. Their relative amounts were in a good agreement within the uncertainties. The relative content of Cr in these samples, obtained by PIGE using 5 MeV proton beam is in agreement with the result obtained by PIXE (Table 2). Cr was the only element visible in P5 and P3 samples by both techniques.
The following LOD values (in mg kg−1) were determined by PIGE (3 MeV protons): 2 (Li), 5 (F), 10–15 (Na), 40–50 (Al), 150–200 (P), 500–600 (Ti), 1000 (Mg), 2000 (Cr), 3000–5000 (Si).
By comparing our results with literature data (impurities’ levels in various uranium samples), similar values were obtained for Li [11]; F, Mg, P, Si, Ti, Cr, and Zr [8]; Na [7–9, 12]; Al [10]; Ni [9, 11]; Cu and Zn [10, 11]. The main analytical technique applied in these reference papers was ICP-MS, which also permitted determination of a larger number of elements in uranium matrix samples, e.g. Ca, K, Mn, Ba, Pb [7] and Be, B, Co, Mo, Cd, Sn, W, Bi [12] at levels of mg kg−1 and tens of mg kg−1, as well as Sm, Eu, Gd, Dy at a level of 0.5 mg·kg−1 [11].
Figure 5 presents an example of the RBS data processing for UO2 sample wrapped in Mylar (C10H8O4) foil (2.5 μm thickness) using RUMP software [29, 30], taking into account the electrical beam charge (Q) value registered by the acquisition software. A good agreement between the experimental and simulated RBS spectra in the case of C (Mylar layer) could be observed, indicating a correct Q value on the target (the fit quality was improved by considering U3O8 presence on the sample surface).
Conclusions
This paper presents an application of selected IBA techniques for the nuclear forensic investigations of minor and trace elements (metallic impurities) present in uranium dioxide samples.
A total number of 15 elements were determined in five uranium (UO2) samples of different enrichment levels, using nondestructive PIXE and PIGE methods. PIXE proved to be suitable for evaluation of eight medium Z elements (Ti, Cr, Fe, Ni, Cu, Zn, and Ga), while PIGE was used as a complementary technique for the determination of lower Z elements (Li, F, Na, Mg, Al, Si, and P). The elements Cr and Ti were determined by both techniques with a similar sensitivity for Ti, and ten times higher sensitivity by PIXE for Cr.
Mass fractions of the impurities determined by us were found to be similar with literature data obtained in principal by mass spectrometry techniques. Their values are situated in the ranges of mg kg−1 (Li, F), tens of mg kg−1 (Li, F, Na, Ga, Zn, Al), hundreds of mg kg−1 (Na, Zn, Al, P, Cu, Ni, Zr), and thousands of mg kg−1 (tenth of percent) (Si, Mg, Zr, Ti, Cr, Fe).
The obtained results proved the suitability of IBA techniques for nuclear forensic investigations of elemental composition of uranium dioxide samples.
References
Portela C (2003) The role of the EU in the non-proliferation of nuclear weapons: the way to Thessaloniki and beyond. PRIF Reports No 65. ISBN:3-933293-83-9. http://www.hsfk.de
Redermeier A (2009) Fingerprinting of nuclear material for nuclear forensics. Esarda Bull 43:71–76
Grant PM, Moody KJ, Hutcheon ID et al (1998) Nuclear forensics in law enforcement applications. J Radioanal Nucl Chem 235:129–132. doi:10.1007/BF02385950
Moody KJ, Grant PM, Hutcheon ID (2005) Nuclear Forensic Analysis. CRC Press, Boca Raton eBook ISBN:978-0-203-50780-3. ISBN 978-0-8493-1513-8
IAEA TECDOC No 1730 (2014) Application of nuclear forensics in combating illicit trafficking of nuclear and other radioactive material
Aggarwal SK (2016) Nuclear forensics: what, why and how? Curr Sci 110(5):782–791
Wallenius M, Mayer K, Ray I (2006) Nuclear forensic investigations: two case studies. Forensic Sci Int 156:55–62
Svedkauskaite-Le Gore J (2008) Development and validation of a method for origin determination of uranium-bearing material. PhD thesis, Vilnius University, University of Physics, Lithuania, JRC Technical Notes, Institute for Transuranium Elements, JRC-ITU-TN-2008/25, Karlsruhe, Germany
Pajo L, Schubert A, Aldave L, Koch L, Bibilashvili YK, Dolgov YN, Chorokhov NA (2001) Identification of unknown nuclear fuel by impurities and physical parameters. J Radioanal Nucl Chem 250(1):79–84
Bürger S, Riciputi LR, Bostick DA (2007) Determination of impurities in uranium matrices by time-of-flight ICP-MS using matrix-matched method. J Radioanal Nucl Chem 274:491–505. doi:10.1007/s10967-006-6930-0
Quemet A, Brennetot R, Chevalier E, Prian E, Laridon AL, Mariet C, Fichet P, Laszak I, Goutelard F (2012) Analysis of twenty five impurities in uranium matrix by ICP-MS with iron measurement optimized by using reaction collision cell, cold plasma or medium resolution. Talanta 99:207–212
Oliveira Junior OP, Sarkis JES (2002) Determination of impurities in uranium oxide by inductively coupled plasma mass spectrometry (ICPMS) by the matrix matching method. J Radioanal Nucl Chem 254(3):519–526
De Souza AL et al (2013) An overview of spectrometric techniques in uranium nuclear fuel grade. Microchem J 106:194–201
Kelkar A, Prakash A, Afzal M, Panakkal JP, Kamath HS (2011) Simultaneous determination of alkali, alkaline earth and transition metal elements in uranium and thorium based nuclear fuel materials by single column ion chromatography. J Radioanal Nucl Chem 287(2):595–601
Natarajan V, Dhawale BA, Rajeswari B, Hon NS, Thulasidas SK, Porwal NK, Godbole SV, Manchanda VK (2008) Determination of metallic impurities in U3O8 using energy dispersive X-ray fluorescence spectrometry. Spectrochim Acta B 63:817–819
Rosenberg RJ, Zilliacus R (1993) Determination of impurities in nuclear fuel element components by neutron activation analysis. J Radioanal Nucl Chem, Articles 169(1):113–124
Erasmus CS, Sellschop JPF, Alfassi ZB (1986) Limits of detection for impurities in uranium-rich matrices by ion-induced nuclear analysis. Nucl Instrum Methods Phys Res B 15:569–572
Ontalba Salamanca MÁ, Gómez-Tubío B, Ortega-Feliu I, Respaldiza MÁ, Luisa de la Bandera M, Ovejero Zappino G, Bouzas A, Gómez-Morón A (2006) External-beam PIXE spectrometry for the study of Punic jewellery (SW Spain): the geographical provenance of the palladium-bearing gold. Nucl Instrum Methods Phys Res B 249(1–2):622–627. doi:10.1016/j.nimb.2006.03.172
Carmona N, Ortega-Feliu I, Gómez-Tubío B, Villegas MA (2010) Advantages and disadvantages of PIXE/PIGE, XRF and EDX spectrometries applied to archaeometric characterization of glasses. Mater Charact 61:257–267
Burducea I, Straticiuc M, Ghita DG, Mosu DV, Calinescu CI, Podaru NC, Mous DJW, Ursu I, Zamfir NV (2015) A new ion beam facility based on a 3 MV Tandetron™ at IFIN-HH, Romania. Nucl Instrum Methods B 359:12–19
Ziegler JF, Biersack JP (2013) SRIM-2013 The stopping and range of ions in matter. http://srim.org/SRIM/SRIMLEGL.htm
Gomez S, Garcia A, Landete-Castillejos T, Gallego L, Pantelica D, Pantelica A, Preoteasa EA, Scafes A, Straticiuc M (2016) Potential of the Bucharest 3 MV Tandetron™ for IBA studies of deer antler mineralization. Nucl Instrum Methods B 371:413–418
Tesmer JR, Nastasi M (1995) Handbook of modern ion beam materials analysis. Materials Research Society, Pittsburg
Pantelica D et al (2016) Characterization of hydrogenated and deuterated silicon carbide films codeposited by magnetron sputtering. Nucl Instrum Methods B 371:322–326. doi:10.1016/j.nimb.2015.10.060
Respaldiza MA, Ager FJ, Carmona A, Ferrer J, García-León M, García-López J, García-Orellana I, Gómez-Tubío B, Morilla Y, Ontalba MA, Ortega-Feliu I (2008) Accelerator-based research activities at “Centro Nacional de Aceleradores”, Seville (Spain). Nucl Instrum Methods B 266(10):2105–2109
Campbell JL, Boyd NI, Grassi N, Bonnick P, Maxwell JA (2010) The Guelph PIXE software package IV. Nucl Instrum Methods B 268:3356–3363
Apostol AI, Pantelica A, Sima O, Fugaru V (2016) Isotopic composition analysis and age dating of uranium samples by high resolution gamma ray spectrometry. Nucl Instrum Methods B 383:103–108
GammaW Software, W. Westmeier, Ebsdorfergrund-Mölln (2007) version 18.3
Doolittle LR (1985) Algorithms for the rapid simulation of Rutherford backscattering spectra. Nucl Instrum Methods B 9(3):344–351. doi:10.1016/0168-583X(85)90762-1
Doolittle LR (1986) A semiautomatic algorithm for rutherford backscattering analysis. Nucl Instrum Methods B 15(1–6):227–231. doi:10.1016/0168-583X(86)90291-0
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Apostol, A.I., Pantelica, A., Ortega-Feliu, I. et al. Ion beam analysis of elemental signatures in uranium dioxide samples: importance for nuclear forensics. J Radioanal Nucl Chem 311, 1339–1346 (2017). https://doi.org/10.1007/s10967-016-5136-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-016-5136-3