Abstract
In this study we tested functionalized AuNPs as an improved drug delivery tool in pancreatic and colon cancer cell lines AR42J and HT-29, using radioimmunoassay method. 68Ga radiolabeled DOTA-Tyr(3)-octreotide, DOTA-NaI(3)-octreotide and DOTA-NT peptides were linked on nanoparticles surface, aiming to increase the peptide density at the tumor site and thus interaction probability, resulting in higher photon signal of 68Ga radioisotope used in positron emission tomography. In vitro ligand-receptor binding evaluation of the radiolabeled compound has showed increased retention of the positron emitter radionuclide 68Ga in the presence of the gold nanoparticles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
68Ga is a short-lived (T 1/2 = 67.83 min) radioisotope obtained after 68Ge/68Ga generator elution, decaying 89 % through positron emission and 11.1 % by electron capture into 68Zn. Although it is available since over 30 years, its clinical research for positron emission tomography (PET) application, has increased only in the last years, after a new class of target-specific radiopharmaceuticals have emerged. 68Ge parent radionuclide has a physical half live of 270.9 days, which enables the use of generator for almost one year at lower costs compared to a cyclotron facility. Despite the higher energy of the positron emission of 68Ga (maximum energy = 1.9 MeV) compared to that of the widely used 18F (maximum energy = 0.6 MeV), 68Ga provides the possibility of PET examinations to the medical centers that do not own an accelerator, or are located at long distance from the [18F]FDG distribution area. 68Ga-based radiopharmaceuticals are generally composed of a chelator agent that bind the radioisotope in complex, and a biologically active targeting moiety, like peptide, hormone or antibody, that bind with high affinity to specific cell surface receptors from the human body.
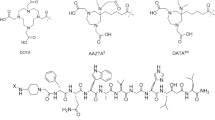
Gold nanoparticles functionalized with radiolabeled peptides have tremendous growth in pharmaceutical field, based on NPs ability to pass the cells membrane by receptor-mediated endocytosis [1]. Nanoparticles binding to biomolecules depend upon ligand containing amine, carboxyl or thiol groups that can be used to covalently attach biomolecules to nanoparticles surface [2, 3]. Coupling methods, often may lead to the degradation or inactivation of biological active sequence of attached molecules. For this purpose, the above mentioned groups should be positioned in terminal position of the molecule or be functionalized on nanoparticles surface [4]. An example of gold nanoparticles functionalized with somatostatin analog-octreotide is illustrated in Fig. 1, where amino terminal group of Tyr(3)-octreotide (TOC) serves as linker between them. Although in vivo application of gold nanoparticles, thiol terminated groups are the most desired one, because they have the highest affinity to noble metal surfaces, amino groups can be used as linker between NPs and peptides but for a limited time.
Somatostatin analog TOC is one of the latest introduced 68Ga labeled peptide in the European Pharmacopoeia, as 68Ga-Edotreotide injection. This cyclic peptide, is targeting with high affinity somatostatin receptor SSTR2 expressed on neuroendocrine tumors. Because moreover, the majority of human tumors do express more than one receptor subtype, other somatostatin analogs have been synthesized, like NaI(3)-octreotide (NOC) who bind with high affinity to SSTR2, SSTR3 and SSTR5 receptors [5].
In the present study, we have successfully synthesized and functionalized gold nanoparticles with three DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) conjugated peptides: neurotensin, NOC and TOC. All peptides were previously radiolabeled with short-lived 68Ga radioisotope obtained from 68Ge/68Ga generator. The radiolabeled complexes were analyzed in terms of radiochemical purity and stability. Nanoparticles physicochemical characterization was performed for determination of hydrodynamic diameter, colloidal stability and maximum absorption corresponding wavelength. Also the successful functionalization of AuNPs was followed through red shift in the absorption spectra of the nanoparticles. Later on, the radiolabeled preparations were incubated on colon cancer cells HT-29 and exocrine pancreatic tumor cells AR42J.
The aim of the study was to evaluate the biological effect of AuNPs upon radioisotope uptake and retention using radioimmunoassay method of LigandTracer Yellow instrument.
Experimental
Synthesis of gold nanoparticles
Nanoparticles preparation was carried out using Turkevich method [6, 7]. 1.25 mL of HAuCl4 × 3H2O solution (20 mM) (Carl Roth GmbH + Co. KG) were dissolved in 1 L distilled water at 80 °C, while kept under vigorous stirring (Ceramic Hot Plate Stirrer—HSC from VELP Scientifica). As reducing agent trisodium citrate dihydrate (16.6 g/L) (Carl Roth GmbH + Co. KG) was added, continuing the stirring process and keeping the temperature constant for at least 20 min., until a deep red stable dispersion was obtained. Sodium citrate also stabilizes the AuNPs by forming a negatively charged double layer [8].
Nanoparticles characterization
Nanoparticles formulation was analyzed for morphology, size distribution and average particle hydrodynamic diameter using dynamic light scattering (DLS) and transmission electron microscopy (TEM) methods. In order to assess nanoparticles formulation stability, electrostatic potential near the NP surface called zeta (ζ) potential was evaluated through PALS (phase analysis light scattering) technique provided by Malvern Nanosizer 90S system.
For further characterization, UV–Vis measurement was performed on Specord 210-Analytic Jena AG spectrophotometer, using 0.75 mL quartz cuvette.
Quality control and peptides radiolabeling with 68Ga radioisotope
DOTA-NaI(3)-octreotide (DOTA-NOC), DOTA-Tyr(3)-octreotide (DOTA-TOC) and DOTA-neurotensin (DOTA-NT) peptides purchased from piChem, Austria, were labeled with short lived positron emitter 68Ga using organic column type 68Ge/68Ga generator (ITG Isotopen Technologien München AG, Germany) and automated labeling module Galigand GAL-102. For radiolabeling process 68Ge/68Ga generator was eluted with 4 mL HCl 0.05 M obtained by dilution of 30 % HCl ultrapure (Merck KGaA) with ultrapure water. From the total eluate volume (4 mL), the first and the last fractions (a total of 2 mL) containing radioactive and metalic impurities and low amount of 68Ga were sent to the waste collecting vial. The midle fraction of the eluate consisting of 2 mL 68GaCl3, which concentrates the most of the 68Ga amount, was then collected in the reaction vial. The available radioactivity for labeling processes was about 630 MBq for DOTA-NOC and between 410 and 430 MBq for DOTA-NT and DOTA-TOC. Depending of the biomolecule used for labeling, ~22 nmol of DOTA-NOC, 35 nmol DOTA-TOC and 24 nmol DOTA-NT were added, previously mixed with 1 M ammonium acetate buffer solution (Sigma Aldrich) to avoid chemical degradation of the peptide [9]. In order to bind 68Ga using DOTA as chelating agent, a temperature of 95 °C was used [10]. The reaction time required for labeling is 5 min, after which the reaction mixture was passed through Phenomenex Strata-X SPE cartridge in order to separate the biomolecule from the free 68Ga and other impurities which pass through the column and go to waste. The column requires previous conditioning with 1 mL ethanol (Chimreactiv, Romania) followed by 1 mL ultrapure water. The peptide is rinsed on the column with a mixture made of 950 μL ultrapure water and 50 μL ethanol. The radiolabeled peptide is then recovered by rinsing the cartridge with 1 mL of ethanol and transferred to a 95 °C preheated vial for ethanol dry evaporation step. In order to ensure physiological pH of the final radiolabeled complex, 1 mL of physiological saline solution is added (0.9 % NaCl solution, B Braun Melsungen).
After labeling, the radiochemical purity was assessed through HPLC using C-18 column, Nucleosil, as stationary phase and acetonitrile and water acidified with 0.1 % TFA as mobile phases [11]. The radiochemical stability of the radioligand complex was evaluated by HPLC in 0.9 % physiological saline solution at 1, 2, 3 and 4 h after the end of the synthesis.
Conjugation of 68Ga-DOTA-TOC, 68Ga-DOTA-NT and 68Ga-DOTA-NOC to AuNPs
For 68Ga-DOTA-peptide-AuNP complex preparation, 1 mL of nanoparticles suspension (having a concentration of 30 mg/L) was mixed with 500 μL 68Ga-DOTA-NOC/TOC/NT (containing 17.5 nmol of TOC, 11.25 nmol of NOC and 12 nmol of NT) and the mixture was stirred for 15 min (450 rot/min.) at room temperature. As we observed from previous experiments, exceeding 15 min stirring can cause nanoparticles irreversible aggregation. No further purification was performed.
In order to assess the nanoparticles payload with the radiolabeled peptides based on gold nanoparticles wavelength shift, additional UV–Vis evaluation was necessary.
Cell culture
Cell lines used in the experiments include the amphicrine pancreatic tumor cell line AR42J (Biochrom GmbH, Berlin, Germany) and the colon cancer cell line HT-29 (Biochrom GmbH, Berlin, Germany). All cells were passaged twice weekly in Ham’s F-12 medium for AR42 cells, supplemented with 20 % fetal bovine serum and 1 % l-glutamine. HT-29 cells were cultured in DMEM medium. All substances were purchased from Biochrom GmbH, Berlin, Germany. The cells were grown at 37 °C in humidified air containing 5 % CO2.
Approximately 5 × 105 pancreatic tumor cells and 4 × 105 colon tumor cells were seeded 24 h prior to use in a local area of the 87 mm diameter petri dish (Nunclon™ Delta Surface 150,350, Thermo Fisher Scientific). In order to use the LigandTracer technology, the dishes were placed slightly tilted during the cells attachment period.
In vitro binding kinetics study
The goal of this experiment was to investigate by comparison the binding of radiolabeled conjugates and functionalized gold nanoparticles on tumor cells. For this purpose we assessed the cell-associated radioactivity as a function of the incubation time using LigandTracer technology (Ridgeview Instruments AB, Sweden) [12, 13].
Prior to each measurement the cell medium was changed with 1 mL of pure medium. The measurements were then performed according to the following steps, as illustrated in Fig. 2:
-
(a)
One data point was acquired for background measurement in order to assess the final retention level of the radiolabeled complex.
-
(b)
3.5 nmol radiolabeled DOTA-TOC/NOC and 2.4 nmol of DOTA-NT were incubated until steady state conditions were reached. An average incubation time of 35 min has been considered the appropriate time to reach steady state [14]. Also because of the short physical half-life of 68Ga radioisotope we limited the measurements to 1 h.
-
(c)
After the equilibrium condition has been occurred the radioactive medium has been removed, followed by cell dish rinsing with 2 mL of DMEM/Ham’s F-12 medium. After the rinsing procedure, the cells were supplied with 1 mL of cell medium.
-
(d)
The final part of the kinetic assay consisted in retention evaluation for a variable time, until the retained radioactive complex activity has dropped up to a saturation level. This level reflects the actual amount of radioisotope restrained on the cell membrane or inside the tumor cells.
Same process has been repeated for gold nanoparticles functionalized with radiolabeled peptides.
Results and discussion
Nanoparticles characterization
The average particle hydrodynamic diameter determined using DLS was 42.57 ± 4 nm as depicted in Fig. 3, ζ potential indicated good colloidal stability with −41.5 ± 6 mV.
68Ga radiolabeled peptides synthesis and quality control
Table 1 summarizes the radiolabeling yield obtained after the synthesis process, also the radiochemical purity evaluated through high performance liquid chromatography (HPLC) system. Radiolabeling yield was calculated dividing the final activity Λ F of pure radiolabeled complex to the sum of activity Λ C retained on SPE cartridge, activity Λ EW of the resulted elution waste (the other 2 mL of 68GaCl3 not used in the synthesis process) and reaction waste activity Λ RW resulted during the synthesis process:
High performance liquid chromatography analysis upon radiochemical purity of labeled conjugates indicated over 98 % chemical purity. As illustrated in Figs. 4, 5 and 6 elution peak of 68Ga-DOTA-TOC appears after t R = 18.61 min, t R = 18.37 min for 68Ga-DOTA-NT and t R = 22.77 min, for 68Ga-DOTA-NOC. 4 h post EOS (end of synthesis), the radiochemical purity maintained over 98 % which indicates the stability of the final products after passing four half-lives of the 68Ga radioisotope, results which are complying with the standards of the European Pharmacopoeia for radiopharmaceuticals preparations.
Gold nanoparticles functionalization
The maximum optical absorbance of the plasmon band of bare gold NP was observed at λ ~524 nm, which correspond to the previous findings in literature [15]. Wavelength shift induced by changes in the refraction index and the surrounding dielectric medium, which occurred as consequence of the interaction between the peptide and the AuNP surface, was evaluated by UV–Vis optical absorption spectra (Fig. 7), showing 2 nm red shift for 68Ga-DOTA-NT-AuNP, 3 nm for 68Ga-DOTA-NOC-AuNP, respectively 5 nm for 68Ga-DOTA-TOC-AuNP.
Uptake and retention assay
In vitro assay aimed to study two characteristics of the peptide-cell interaction process: cellular uptake and cellular retention.
The first part of the binding trace consists of uptake measurement, which corresponds to radiolabeled conjugates incubation time. The measurements results were decay corrected to the half-life of 68Ga (67.83 min) and in order to compare the two measurements performed on every peptide (one for 68Ga radiolabeled peptide and one for gold nanoparticles functionalized with same radiolabeled peptide) the final data were normalized.
The second part of the binding trace consists of retention evaluation by removing the hot medium from cells, rinsing the cell dish with fresh medium and resume the measurement in order to assess the amount of radioactivity detected due to peptide attachment to its specific receptor from the cell surface. All studies used 3.5 nmol of radiolabeled peptide, different radioligand volumes being needed to ensure this quantity. Parameters used in the uptake/retention process are summarized in Table 2.
The binding kinetics of both cellular uptake and retention of the somatostatin analog Tyr(3)-octreotide are presented in Fig. 8. As one can see the uptake slope of the radiolabeled complexes are almost the same, leading the conclusion that gold nanoparticles do not interfere in the uptake process of the radioisotope. In return, the retained radioactivity difference between the two conjugates is around 50 %, from 10 ± 3 % in the case of 68Ga-DOTA-TOC up to 60 ± 2 % 68Ga-DOTA-TOC-AuNP.
As in the case of TOC, NOC peptide complexes have the same trend line for uptake but functionalized gold nanoparticles have a 35 % elevated radioligand retention compared to 68Ga-DOTA-NOC, as depicted in Fig. 9.
At the beginning, for radiolabeled neurotensin conjugates we performed the assay on HT-29 cell line, but the results showed only 10 % improved retention so we also evaluate the radioisotope accumulation on AR42J cell line (Fig. 10). For the second tumor cell line tested, the same retention level could be observed (Fig. 11). Because neurotensin have multiple NH2 groups in its structure, not just in the terminal position, we assumed that there is a possibility that both linker and acceptor implicated in AuNP-neurotensin interaction are immobilized on the surface of different nanoparticles and induce their assembly [16].
Conclusions
In summary this in vitro binding kinetics study evaluated the ability of gold nanoparticles to increase the available 68Ga positron emitter radioisotope inside tumor cells, using together AuNPs properties to pass cells membrane by endocytosis, and radiolabeled peptides specific tumor targeting properties.
Gold nanoparticles functionalized with 68Ga-DOTA-NOC and 68Ga-DOTA-TOC showed over 60 % radionuclide retention after hot medium extraction, with an average of 35 %, respectively 50 % improved retention in comparison with 68Ga-DOTA-NOC and 68Ga-DOTA-TOC conjugates. In contrast, neurotensin functionalized AuNPs showed 10 % slightly elevated retention in comparison with 68Ga-DOTA-NT.
These results are attributed to receptors mediated uptake of the nanoparticles inside the tumor, and their possibility to bind several peptides on their surface, increasing the available radioisotope inside the tumor cells. We anticipate that in vivo application of these functionalized AuNPs, with proper coating, might improve the quality of the PET images.
Further studies need to be performed upon these functionalized nanoparticles toxicity and also their possible interaction with peptides properties to bind on specific receptors site.
References
Zhang S, Li J, Lykotrafitis G, Bao G, Suresh S (2009) Size-dependent endocytosis of nanoparticles. Adv Mater 21:419–424
De Jong WH, Borm PJ (2008) Drug delivery and nanoparticles: applications and hazards. Int J Nanomed 3:133–149
Tiwari PM, Vig K, Dennis VA, Singh SR (2011) Functionalized gold nanoparticles and their biomedical applications. Nanomaterials 1:31–63
Sperling RA, Parak WJ (2010) Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos Trans R Soc Lond A 368:1333–1383
Wild D, Schmitt JS, Ginj M et al (2003) DOTA-NOC, a high-affinity ligand of somatostatin receptor subtypes 2, 3 and 5 for labelling with various radiometals. Eur J Nucl Med Mol Imag 30:1338–1347
Turkevich J, Stevenson PC, Hillier J (1951) A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc 11:55–75
Kimling J, Maier M, Okenve B, Kotaidis V, Ballot H, Plech A (2006) Turkevich method for gold nanoparticle synthesis revisited. J Phys Chem 110:15700–15707
Ji X, Song X, Li J, Bai Y, Yang W, Peng X (2007) Size control of gold nanocrystals in citrate reduction: the third role of citrate. J Am Chem Soc 129:13939–13948
Patel K, Borchardt RT (1990) Chemical pathways of peptide degradation. II. Kinetics of deamidation of an asparaginyl residue in a model hexapeptide. Pharm Res 7:703–711
Sosabowski JK, Mather SJ (2006) Conjugation of DOTA-like chelating agents to peptides and radiolabeling with trivalent metallic isotopes. Nat Protoc 1:972–976
Pătraşcu I, Niculae DA, Lungu VA, Ursu I, Iliescu M, Tuta C, Antohe A (2011) The purification and the quality control of 68Ga eluates from 68Ge/68Ga generator. Rom Rep Phys 63:988–996
Bjorke H, Andersson K (2006) Measuring the affinity of a radioligand with its receptor using a rotating cell dish with in situ reference area. Appl Radiat Isot 64:32–37
Bjorke H, Andersson K (2006) Automated, high-resolution cellular retention and uptake studies in vitro. Appl Radiat Isot 64:901–905
Ahmadi F, Dabirian S, Faizi M, Tabatabai SA, Beiki D, Shahhosseini S (2014) Optimum conditions of radioligand receptor binding assay of ligands of benzodiazepine receptors. Iran J Pharm Res 13:79–86
He YQ, Liu SP, Kong L, Liu ZF (2005) A study on the sizes and concentrations of gold nanoparticles by spectra of absorption, resonance Rayleigh scattering and resonance non-linear scattering. Spectrochim Acta A 61:2861–2866
Schone D, Schade B, Bottcher C, Koksch B (2015) Impact of multivalent charge presentation on peptide–nanoparticle aggregation. Beilstein J Org Chem 11:792–803
Acknowledgments
The work has been Funded by the Sectoral Operational Programme Human Resources Development 2007–2013 of the Ministry of European Funds through the Financial Agreement POSDRU/159/1.5/S/134398 and PN II Partnerships in priority areas programme, MEN-UEFISCDI, Contract No. 228/2014.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chilug, L.E., Leonte, R.A., Patrascu, M.E.B. et al. In vitro binding kinetics study of gold nanoparticles functionalized with 68Ga-DOTA conjugated peptides. J Radioanal Nucl Chem 311, 1485–1493 (2017). https://doi.org/10.1007/s10967-016-5075-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-016-5075-z