Abstract
Earlier studies have shown that modification of the octapeptide octreotide in positions 3 and 8 may result in compounds with increased somatostatin receptor affinity that, if radiolabelled, display improved uptake in somatostatin receptor-positive tumours. The aim of a recent research study in our laboratory was to employ the parallel peptide synthesis approach by further exchanging the amino acid in position 3 of octreotide and coupling the macrocyclic chelator DOTA(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) to these peptides for labelling with radiometals like gallium-67 or -68, indium-111, yttrium-90 and lutetium-177. The purpose was to find radiopeptides with an improved somatostatin receptor binding profile in order to extend the spectrum of targeted tumours. A first peptide, [111In,90Y-DOTA]-1-Nal3-octreotide (111In,90Y-DOTA-NOC), was isolated which showed an improved profile. InIII-DOTA-NOC exhibited the following IC50 values (nM) when studied in competition with [125I][Leu8, d-Trp22, Tyr25]somatostatin-28 (values for YIII-DOTA-NOC are shown in parentheses): sstr2, 2.9±0.1 (3.3±0.2); sstr3, 8±2 (26±1.9); sstr5, 11.2±3.5 (10.4±1.6). Affinity towards sstr1 and 4 was very low or absent. InIII-DOTA-NOC is superior to all somatostatin-based radiopeptides having this particular type of binding profile, including DOTA-lanreotide, and has three to four times higher binding affinity to sstr2 than InIII,YIII-DOTA-Tyr3-octreotide (InIII,YIII-DOTA-TOC). In addition, [111In]DOTA-NOC showed a specific and high rate of internalization into AR4-2J rat pancreatic tumour cells which, after 4 h, was about two times higher than that of [111In]DOTA-TOC and three times higher than that of [111In]DOTA-octreotide ([111In]DOTA-OC). The internalized radiopeptides were externalized intact upon 2 h of internalization followed by an acid wash. After 2–3 h of externalization a plateau is reached, indicating a steady-state situation explained by reactivation of the receptors followed by re-endocytosis. Biodistribution studies in CA 20948 tumour-bearing rats showed rapid clearance from all sstr-negative tissues except the kidneys. At 4 h the uptake of [111In]DOTA-NOC in the tumour and sstr-positive tissues, such as adrenals, stomach and pancreas, was three to four times higher than that of [111In]DOTA-TOC. Differential blocking studies indicate that this is at least partially due to the uptake mediated by sstr3 and sstr5. These very promising preclinical data justify the use of this new radiopeptide for imaging and potentially internal radiotherapy studies in patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radiopeptides are becoming of increasing interest in tumour targeting for either the localization or the internal radiotherapy of neoplasms [1, 2, 3, 4, 5, 6, 7, 8, 9]. Analogues of the somatotropin release inhibiting factor (SRIF), somatostatin, radiolabelled with a variety of gamma-, positron- and beta-emitters, are the prototypes of such peptides. Somatostatin is a cyclic peptide hormone that occurs naturally in two bioactive molecular forms: somatostatin-14 and its N-terminally extended form, somatostatin-28. It exerts different biological effects in different parts of the body such as the brain, the pituitary, the pancreas, the gut and some components of the immune system. The effects include inhibition of hormone secretion and modulation of neurotransmission and cell proliferation. These actions are mediated by specific, G-protein-coupled receptors. Today five different somatostatin receptor subtypes have been characterized and cloned (sstr1–5). They are responsible for different biological responses. As some of these receptors are over-expressed in several human tumours, especially neuroendocrine tumours and their metastases, these tumours can be visualized in vivo by radiometal chelator conjugated somatostatin analogues like [111In-DTPA-d-Phe1]-octreotide (OctreoScan) [10]. It has also been shown that somatostatin receptor scintigraphy using this agent is the most sensitive method for localization of primary and metastatic disease in endocrine pancreatic tumours and carcinoids except insulinomas [11]. New conjugates may show higher sensitivity with regard to the localization of tumours and metastases, e.g. 99mTc-depreotide is registered in many countries and appears to show good performance in the evaluation of solitary pulmonary nodules [12], in breast tumours and even in melanoma [13]. On the other hand, a recent study comparing OctreoScan with 99mTc-depreotide in 44 patients with neuroendocrine tumours showed that the 111In-labelled peptide yielded a far higher detection rate for neuroendocrine tumours, especially for liver metastases [14]. 111In]DOTA-TOC and [90Y]DOTA-TOC (DOTA = 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) have been shown to be effective targeting and therapeutic agents in animal models and patients [15, 16, 17, 18, 19, 20, 21, 22, 23]. In addition, replacement of the alcohol group at the C-terminus of the octapeptide by a carboxylic acid group led to increased sstr2 affinity [24], and [177Lu-DOTA]-d-Phe1-Tyr3-Thr8-octreotide ([177Lu]DOTA-TATE) showed higher tumour uptake than [111In-DTPA]-octreotide in six patients with somatostatin receptor-positive tumours [25]. These new radiopeptides show distinctly higher sstr2 affinity compared with OctreoScan. Nevertheless, they bind with high affinity only to sstr2: their affinity to sstr5 is low, and that to sstr3 almost negligible; no affinity of these new compounds was found to sstr1 and sstr4. Although the majority of tumours studied with radiolabelled somatostatin analogues express mainly sstr2 [26], recent literature data indicate that also sstr1 and sstr3–5 may be present in some human tumours. For example, in binding studies using [125I]-RC-160 (d-Phe-Cys-Tyr-d-Trp-Lys-Val-Cys-Trp-NH2; K D=6.55 nM) as the radioligand, Halmos et al. [27] found somatostatin receptors to be present on 76% of human epithelial ovarian cancers. By use of in situ hybridization, Reubi et al. found m-RNAs of sstr1, 2 and 3 in a variety of human tumours, including GH-adenoma [28]. Forssell-Aronsson et al. reported the lack of sstr2 (except for medullary thyroid carcinoma) with Northern blot in most of the thyroid tumours studied in 68 patients. Nevertheless, all tumour types regularly expressed sstr1, sstr3, sstr4 and sstr5 [29]. Raderer et al. could visualize primary pancreatic adenocarcinoma in vivo with [111In]DOTA-lanreotide (LAN; d-2-Nal-Cys-Tyr-d-Trp-Lys-Val-Cys-Thr-NH2) but not with [111In]DTPA-octreotide [30]. Traub et al. reported [111In]DOTA-LAN to have a high sensitivity for detection of lung cancer [31]. However, their claim that this peptide targets sstr2–5 with high affinity and sstr1 with lower affinity could not be confirmed by Reubi et al. [24]. To extend the biological activity profile of radiolabelled somatostatin analogues, we started a programme to synthesize radiopeptides with affinity to all somatostatin receptor subtypes in order to potentially extend the spectrum of accessible tumours in diagnosis and internal radiotherapy. A first compound resulted from a parallel synthesis approach exchanging the amino acid in position 3 of octreotide; this led to a radiopeptide, [111In/90Y-DOTA]-1-Nal3-octreotide ([111In/90Y]DOTA-NOC), which had improved affinity to sstr2 and high affinity to sstr3 and sstr5 when compared with our lead compound [111In/90Y]DOTA-TOC.
Here we present the preclinical evaluation of this peptide with regard to binding affinity, rate of internalization and biodistribution in a tumour-bearing rat model and compare its properties with those of [111In/90Y-DOTA0-Tyr3]-octreotide and [111In/90Y-DOTA0]-octreotide.
Materials and methods
All chemicals were obtained from commercial sources and used without further purification. H-Thr(tBu)-ol-(2-chloro-trityl)-resin was obtained from Advanced ChemTech (Giessen, Germany) and Fmoc (9-fluorenylmethoxycarbonyl) amino acids were purchased from NovaBiochem AG (Läufelfingen, Switzerland), Bachem (Bubendorf, Switzerland) and Neosystems (France). 111InCl3 was obtained from Mallinckrodt Medical (Petten, The Netherlands). The prochelator DOTA(tBu)3 (4,7,10-tricarboxymethyl-tert-butyl ester 1,4,7,10-tetraazacyclododecane-1-acetate) was synthesized according to Heppeler et al. [32] or purchased from Macrocyclics (Richardson, Tex., USA). The reactive side chains of the amino acids were masked with one of the following groups: Cys, acetamidomethyl; Lys, t-butoxycarbonyl; Thr, t-butyl; Trp, t-butoxycarbonyl. Analytical reversed-phase high-performance liquid chromatography (RP-HPLC) was carried out on a Hewlett Packard 1050 HPLC system (Waldbronn, Germany) equipped with a multi-wavelength detector and a flow-through Berthold LB506C1 gamma-detector. Preparative HPLC was done on a Bischof HPLC system (Metrohm AG, Switzerland) with HPLC-pumps 2250 and a Lambda 1010 UV detector (Metrohm AG, Switzerland). CC250/4 Nucleosil 120-3C18 columns from Macherey-Nagel were used for analytical HPLC, and a VP250/21 Nucleosil 200-5C15 column for preparative HPLC. The gradient systems consisted of mixtures of water with 0.1% trifluoroacetic acid (TFA) (solvent A) and acetonitrile (solvent B). Quantitative gamma-counting was performed on a COBRA 5003 gamma-system well counter from Packard Instrument Company (Switzerland). Electrospray ionization mass spectrometry (ESI-MS) was carried out with a Finnigan SSQ 7000 spectrometer (Bremen, Germany).
Synthesis
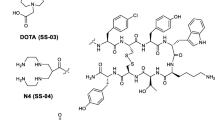
The peptide-chelator conjugates were synthesized by standard Fmoc solid phase synthesis [33] on 2-chlorotritylchloride resin (substitution 0.8 mmol/g) on a Rink peptide-synthesizer Switch 24 (RinkCombichem Technologies, Bubendorf, Switzerland), according to the general procedure described previously [32]. The last step was the coupling of the prochelator DOTA(tBu)3 to the N-terminus of the peptide. Cleavage of the fully protected conjugates from the resin, oxidative cyclization using iodine, deprotection and HPLC purification led to compounds 1–3 (Table 1, Fig. 1), which could be labelled with "cold" or radioactive In3+ or Y3+ (InIII, YIII). All compounds were lyophilized after purification and characterized by ESI-MS and RP-HPLC. In each case the MS spectra consisted of a major [M+2Na]2+ ion peak and some other smaller peaks corresponding to [M+Na]+ and [M+3Na]3+ ions. All peptide-chelator conjugates had a purity >95% confirmed by RP-HPLC. The results are given in Table 1. The peptide-DOTA conjugates obtained were designated DOTA-NOC [DOTA0-d-Phe1-1-Nal3]-octreotide), DOTA-OC [DOTA0-octreotide]) and DOTA-TOC [DOTA0-Tyr3]-octreotide).
The DOTA-SRIF analogues were complexed with InCl3 (anhyd.) and Y(NO3)3·5 H2O, using the following procedure: ca. 20 μg of the respective DOTA-peptide was heated along with 1.5 eq. of the corresponding metal salt in 150 μl 0.2 M sodium acetate buffer (pH 5) for 25 min. After cooling, 20 μl 0.1 M DTPA (pH 5) was added to complex free metal ions. This mixture was loaded onto a SepPak C18 cartridge (Millipore, Switzerland), activated using 5 ml MeOH followed by 10 ml H2O. [MIII(DTPA)]2− was washed from the cartridge using water. The MIII-DOTA-peptide was eluted with methanol and obtained in >97% purity after evaporation of methanol. The radiopeptides were synthesized according to Heppeler et al. [32] and obtained in >99% radiochemical purity at specific activities of >37 GBq/μmol peptide.
For internalization experiments, the DOTA-peptides were labelled to a specific activity of about 37 GBq/μmol peptide; excess InCl3 was then added and the mixture was purified on a SepPak C18 cartridge as described above to afford [111In/InIII]DOTA-peptides.
Determination of the somatostatin receptor affinity profiles
Cells stably expressing human sstr1–5 were grown as described previously [24]. All culture reagents were supplied by GIBCO/BRL and Life Technologies (Grand Island, N.Y.). Cell membrane pellets were prepared and receptor autoradiography was performed on pellet sections (mounted on microscope slides) as described in detail previously [24]. For each of the tested compounds, complete displacement experiments were performed with the universal somatostatin radioligand [125I][Leu8,d-Trp22,Tyr25]somatostatin-28 using increasing concentrations of the unlabelled peptide ranging from 0.1 to 1,000 nM. Somatostatin-28 was run in parallel as control using the same increasing concentrations. IC50 values were calculated after quantification of the data using a computer-assisted image processing system. Tissue standards (autoradiographic [125I]microscales Amersham, UK) containing known amounts of isotopes, cross-calibrated to tissue-equivalent ligand concentrations, were used for quantification [24]. The concentrations of the peptide solutions were measured by UV-spectroscopy (εNOC,280 mm=9,855).
Cell culture and radioligand internalization studies
Sst2 receptor expressing AR4-2J cells were obtained from Novartis Pharma (Basel, Switzerland). The AR4-2J cell line was maintained by serial passage in mono-layers in Dulbecco's Modified Eagle's Medium (DMEM), supplemented with 10% fetal bovine serum, amino acids, vitamins and penicillin-streptomycin, in a humidified 5% CO2/air atmosphere at 37°C. Viability of the cells and cell numbers were counted under a microscope with a "Neubauer's counting chamber". For all cell experiments, the cells were seeded at a density of 0.8–1.1 million cells/well in six-well plates and incubated overnight with internalization buffer to obtain a good cell adherence. The loss of cells during the internalization experiments was below 10%. When different radiolabelled peptides were compared in cell experiments, the same cell suspension-containing plates were used. Furthermore, the internalization rate was linearly corrected to 1 million cells/well in all cell experiments.
Medium was removed from the six-well plates and cells were washed once with 2 ml of internalization buffer (DMEM, 1% fetal bovine serum, amino acids and vitamins, pH 7.4). Furthermore, 1.5 ml internalization buffer was added to each well and the plates were incubated at 37°C for about 1 h. Thereafter approximately 500,000 cpm or 0.02 MBq/well 111In/115In-labelled peptides (2.5 pmol/well) to a final concentration of 1.67 nM were added to the medium and the cells were incubated at 37°C for the indicated time periods in triplicate. Internalization was also studied using three different concentrations of [111In/115In]DOTA-NOC (0.15 pmol/well or 0.1 nM, 2.5 pmol/well or 1.67 nM, and 10 pmol/well or 6.67 nM). To determine non-specific membrane binding and internalization, cells were incubated with radioligand in the presence of 1 μM octreotide. Cellular uptake was stopped by removing medium from the cells and by washing twice with 1 ml of ice-cold phosphate-buffered saline (PBS). Acid wash for 10 min with a 0.1 M glycine buffer pH 2.8 on ice was also performed twice. This was shown previously to be sufficient to remove >90% of receptor-bound radioligand. This procedure was performed to distinguish between membrane-bound (acid-releasable) and internalized (acid-resistant) radioligand. Finally, the cells were treated with 1 N NaOH. The culture medium, the receptor-bound and the internalized fraction were measured radiometrically in a gamma-counter (Packard, Cobra II).
Radioligand externalization studies
AR4-2J cells (1 million) were incubated with 2.5 pmol/well or 1.67 nM [111In/115In]-labelled DOTA-NOC, DOTA-TOC or DOTA-OC for 120 min, then the medium was removed and the wells were washed twice with 1 ml ice-cold PBS. In each experiment an acid wash for 10 min on ice with a glycine buffer of pH 2.8 was performed to remove the receptor-bound ligand. Cells were then incubated again at 37°C with fresh externalization buffer (DMEM containing 1% fetal bovine serum pH 7.4). After different time points the external medium was removed for quantification of radioactivity in a gamma-counter and replaced with fresh 37°C externalization medium. Internalized ligand was extracted in 1 N NaOH, removed and quantified in a gamma-counter. The recycled fraction was expressed as a percentage of the total internalized amount per 1 million cells, and the stability of the externalized peptides was determined using HPLC after removal of the solvent by a centrifugal evaporator.
Biodistribution
Animal experiments were performed in compliance with the regulations of our institutions and with generally accepted guidelines governing such work. Male Lewis rats (200–250 g) bearing the CA20948 pancreatic tumour (0.4–3.5 g) were used in the experiments. Rats were injected under ether anaesthesia with 2–3 MBq of 0.34 nmol (0.5 μg total peptide mass) [111In]DOTA-NOC in 0.5 ml saline into the dorsal vein of the penis. At several time points, rats were sacrificed under ether anaesthesia. Organs and blood were collected and the radioactivity in these samples was determined using a gamma-counter.
In order to determine the non-specific uptake of the radiopeptides, rats were injected with 0.5 mg octreotide in 0.5 ml saline as a co-injection with the radioligand.
To study the sstr2-, 3- and 5-related specific uptake of [111In]DOTA-NOC in the SRIF receptor-positive tissues, blocking studies were designed with two different somatostatin analogues: DTPA-TATE (sstr2-selective ligand) and InIII-DOTA-NOC (sstr2, 3, and 5 affinity). Twenty-five micrograms of these peptides was co-injected with 2–3 MBq [111In]DOTA-NOC (0.34 nmol in 0.5 ml saline) into the dorsal vein of the penis of non-tumour-bearing male Lewis rats. Rats were sacrificed at 24 h and the organs of interest collected and counted for radioactivity.
Statistical methods
Student's t test was used to determine statistical significance. Differences at the 95% confidence level (P<0.05) were considered significant.
Results
Synthesis and radiolabelling
The three DOTA-coupled octapeptides (Fig. 1) were obtained by parallel synthesis on a trityl chloride resin and are part of a small library. The peptides not reported here will be discussed in a more comprehensive chemistry publication. The overall yield of the DOTA peptides was about 30% based on the first Fmoc cleavage. The peptides were prepared and purified to >95% purity by HPLC analysis.
Uncomplexed and metal ion-complexed DOTA peptides were characterized by HPLC, by ESI-MS and by the retained affinity to the somatostatin receptors. Some selected analytical data are given in Table 1. Labelling was performed in acetate buffer (pH 5, 0.4 M) by heating at 95°C for 25 min, affording labelling yields with 111In and 90Y of >99% at a specific activity of >37 GBq/μmol peptide. The HPLC elution profile of the three 111In-labelled peptides is shown in Fig. 2. The respective RF values are 17.03 min for [111In]DOTA-TOC, 18.83 min for [111In]DOTA-OC and 20.88 min for [111In]DOTA-NOC (for a gradient see the footnote to Table 1), indicating that this is the order of increasing lipophilicity.
Receptor binding and affinity profiles
Table 2 shows the IC50 values of the three radiopeptides studied in this work as their YIII (InIII) complexed versions and of YIII-DOTA-LAN for the five somatostatin receptor subtypes. The values were obtained by performing complete displacement experiments with the universal somatostatin radioligand [125I][Leu8, d-Trp22, Tyr25]-somatostatin-28 on membranes from cells expressing the receptor subtypes and were compared with the data for somatostatin-28.
All compounds bound specifically to sstr2 with IC50 values between 3 and 23 nM. High specific binding affinities to sstr3 were also found for InIII-DOTA-NOC (IC50=8±2 nM), YIII-DOTA-NOC (IC50=26±1.9 nM) and YIII-DOTA-OC (IC50=27±8 nM). YIII-DOTA-TOC and YIII-DOTA-LAN showed only very low affinities to sstr3 (IC50 ≥300 nM).
All metallopeptides also showed specific binding to sstr5; YIII-DOTA-TOC bound with IC50=204±92 nM and YIII-DOTA-OC with IC50=58±22 nM whereas YIII-DOTA-LAN (IC50=16.3±3.4 nM) and YIII-DOTA-NOC (IC50=10.4±1.6 nM) showed rather high affinities to sstr5.
In vitro internalization studies in AR4-2J cells
Figure 3 shows the results in respect of the time-dependent internalization of [111In/115In]DOTA-NOC, [111In/115In]DOTA-TOC and [111In/115In]DOTA-OC into AR4-2J rat pancreatic tumour cells during a 240-min incubation period at 37°C. About 85%–95% of totally internalized ligand was specifically internalized. At 30 min and 1.67 nM concentration, [111In]DOTA-NOC showed 7.2%±1.0% specific cell uptake of the total activity administered which increased to 24.8%±1.6% uptake at 4 h. A tendency to reach a plateau was found at 24 h for all three peptides (data not shown). The uptake of [111In]DOTA-OC at 30 min was only 1.0%±0.2% and increased to 7.5%±0.3% at 4 h, whereas [111In]DOTA-TOC showed 2.0%±0.7% internalization at 30 min, rising to 11.6%±0.8% at 4 h. The percentage of internalized peptide measured at 30 min as a function of concentration is shown in Fig. 4.
Comparison of the internalization rate of [111In]DOTA-NOC (■), [111In]DOTA-TOC (●) and [111In]DOTA-OC (▲) into AR4-2J cells. Values and standard deviations are the result of three independent experiments with triplicates in each experiment and are expressed as specific internalization (percentage of dose added to 1 million cells at 1.67 nM concentration, 37°C)
Comparison of the percentage (± standard deviation) of radiopeptide internalized into AR4-2J cells (1 million cells, 37°C) after 30 min with different radiopeptide concentrations. Symbols are as in Fig. 3
The rate of externalization is shown in Fig. 5. In these experiments, 111In labelled peptides were allowed to internalize for 120 min; cells were then washed twice with PBS before removing the receptor-bound ligand with the glycine buffer. Medium was then added and removed after 10 min, 30 min, 60 min, 120 min and 240 min and measured for radioactivity. Up to 60 min, the three peptides showed insignificant differences in the externalization rate. Thereafter the extent of externalized ligand was highest for [111In]DOTA-OC, with about 60% at 4 h compared with 47%±4% for [111In]DOTA-NOC and 43%±2% for [111In]DOTA-TOC. At 4 h the externalization curve of all peptides showed a plateau. Study of the chemical structure of the externalized [111In]DOTA-NOC and [111In]DOTA-TOC by HPLC gave no indication of metabolites. The peptides externalized were intact.
Comparison of the externalization rate (± standard deviation) after 120-min internalization of the three radiopeptides. Symbols are as in Fig. 3
Biodistribution studies in rats
The 4-h, 24-h and 48-h uptake values of [111In]DOTA-NOC and [111In]DOTA-TOC in sst receptor-positive organs, including pancreas, adrenals, pituitary, stomach and CA 20948 rat pancreatic tumour, as well as the kidneys, liver, spleen, femur and blood are shown in Table 3. Both radiopeptides displayed rapid blood clearance with less than 0.02% ID/g remaining in the blood at 4 h. There was also fast clearance from all sstr-negative tissues except the kidneys. The excretion of both peptides was mainly by the kidneys. [111In]DOTA-NOC had a significantly higher uptake than [111In]DOTA-TOC in all sstr-positive tissues at all time points, e.g. at 4 h (tumour, 3.9%±0.6% ID/g vs 1.15%±0.16% ID/g; pancreas, 7.75%±1.4% ID/g vs 2.57%±0.08% ID/g; adrenals, 7.43%±0.42% ID/g vs 3.62%±0.14% ID/g; pituitary, 1.66%±0.20% ID/g vs 1.48%±0.07% ID/g).
To estimate the uptake in sstr-positive organs which may be due to receptor subtype expression other than sstr2, in vivo blocking studies were performed in normal rats using different blocking agents like [DTPA0-Tyr3-Thr8]-octreotide (DTPA-TATE), an sstr2-specific ligand (IC50=3.9±1 nM), and InIII-DOTA-NOC (IC50: sstr2=2.9±0.1 nM, sstr3=8±2 nM, sstr5=11.2±3.5 nM). In the adrenals, blocking with only 25 μg of InIII-DOTA-NOC showed a very efficient reduction of about 95% whereas 25 μg of the sstr2-selective ligand DTPA-TATE resulted in only about 75% blocking.
A significantly higher blocking effect was also found with InIII-DOTA-NOC in the pancreas, pituitary and stomach (data not shown).
Discussion
Receptor scintigraphy with 111In-DTPA-octreotide has become the "gold standard" for the localization, staging and management of neuroendocrine tumours [34]. The high sensitivity of somatostatin receptor scintigraphy and its ability to change the management of patients with neuroendocrine tumours has been demonstrated in several studies [35, 36, 37, 38, 39]. The same agent has been used for receptor-mediated radionuclide therapy with some success if injected in high doses (6 GBq every 4 weeks) for total doses of up to about 100 GBq [40, 41, 42]. This agent only binds with reasonably high affinity to sstr2 and with low affinity to sstr5 [24]; in addition, 111In is not an ideal therapeutic radionuclide. Therefore several groups have developed agents based on somatostatin for improved targeting with positron emitters like 68Ga, 64Cu and the gamma emitter 111In, and most importantly for labelling with therapeutic radionuclides like 90Y and 177Lu. The most successful peptides have been [Tyr3]-octreotide (TOC) and [Tyr3, Thr8]-octreotide (octreotate = TATE) coupled to the macrocyclic chelator DOTA (DOTA-TOC, DOTA-TATE) [32, 43, 44, 45]. These agents bind with high affinity only to sstr2. Although sstr2 is probably the most abundantly expressed SRIF receptor in human cancer [26], subtypes sstr1, sstr3, sstr4 and sstr5 may also be of interest. So, our approach is focussed on the identification of analogues with a pan-somatostatin binding profile carrying functional groups for radiolabelling [46] and we have started a programme to use parallel synthesis methods to produce DOTA-coupled octapeptides with the aim of improving the affinity to subtypes other than 2 while maintaining the sstr2 affinity. In a series of compounds we found that [DOTA0-1-naphthyl3]-octreotide shows promising properties owing to its high affinity to sstr2, with an IC50 value of 3.3±0.2 nM if complexed to YIII and 2.9±0.1 nM if complexed to InIII, which is three- to fourfold higher than the corresponding value for YIII-DOTA-TOC (IC50=11.4±1.7 nM). The new metallopeptides are equipotent to somatostatin-28 on sstr2 and a factor of 7 more potent than YIII-DOTA-LAN. Moreover, they show good affinity to sstr3 and are significantly more potent at sstr5 than YIII-DOTA-LAN. These data do not confirm the pan-somatostatin-like binding affinities of DOTA-LAN published earlier by Smith-Jones et al. [47]. In addition, InIII- and YIII-DOTA-NOC show the highest affinities on sstr3. A metal ion dependence is found at sstr3 and sstr4, InIII-DOTA-NOC being about three to four times more potent than YIII-DOTA-NOC. A potential explanation for this phenomenon is the difference in the coordination geometry of the two DOTA-metalIII complexes, which was documented using 1H-NMR spectroscopy and X-ray crystallography [48]. YIII-DOTA-OC and YIII-DOTA-NOC are equipotent on sstr3 and about a factor of 6 less potent than the endogenous ligand. There is no metal ion dependence in affinity to sstr5.
Cell uptake and release
In order to obtain a defined and homogeneous metallopeptide, [111In]DOTA-NOC was complexed with "cold" InIII to yield [111In, 115In]DOTA-NOC. At 4 h of internalization, [111In/115In]DOTA-NOC showed a factor of 2 higher specific cell uptake than [111In/115In]DOTA-TOC and a factor of about 3 higher than [111In/115In]DOTA-OC at 1.67 nM peptide concentration per 1 million cells. The difference was even more pronounced at 0.1 nM concentration (Fig. 4). This order follows the receptor affinity of the three radiopeptides, indicating that receptor affinity is the major factor determining the rate of internalization.
As the addition of excess cold octreotide inhibits 90% of the uptake, it can be considered as specific and receptor mediated. In the time interval of the study, no steady state was reached, but the distinct leveling off of [111In/115In]DOTA-NOC uptake indicates that steady state was closely approached; we explain this by the onset of efflux of radiopeptides that were shown to be structurally intact.
If, upon internalization of the radioligand for 2 h, the cells were exposed to the culture medium, a time-dependent efflux of the radiopeptides could again be observed, indicating rapid recycling to the extracellular medium. A steady state was reached already after 2–3 h of release (Fig. 5). This is in agreement with data that we have published previously on [67Ga]NODAGA-TOC [49]. We interpret this as beginning reactivation of the receptors by the intact externalized peptides and concomitant re-endocytosis. The finding that the weakest binder [111In]DOTA-OC apparently shows the most efficient externalization fits with this explanation. The fact that the externalized peptides are still intact upon release is another indication that this is the correct interpretation. It is also in keeping with the conclusion drawn from data obtained by Koenig et al. [50].
Biodistribution studies
The biodistribution studies in CA20948-bearing tumour rats demonstrated superior uptake of [111In]DOTA-NOC compared with [111In]DOTA-TOC at 4 h, 24 h and 48 h in receptor-positive normal tissues (except the pituitary) and the tumour. An estimated area under the curve showed an improvement of approximately 2.5-fold in the tumour. This improvement is likely due to the improved sst2, sst3 and sst5 receptor affinity and the significantly faster rate of internalization, as exemplified in the AR4-2J cell line. The octreotide co-injection experiment demonstrated that the uptake is specific and receptor mediated.
Radiometal labelled radiopeptides show high and persistent kidney uptake, limiting their therapeutic potential. One of the goals in the design of new somatostatin-based radioligands is to reduce their uptake in the kidney. Indeed, the tumour-to-kidney ratio of [111In]DOTA-NOC is improved 2.5-fold compared with [111In]DOTA-TOC.
Despite the distinctly higher lipophilicity of [111In]DOTA-NOC over [111In]DOTA-TOC, the uptake in the liver and the intestines is surprisingly low. In addition, the long residence time of the new radiopeptide in the tumour indicates that it is not only suitable for imaging but also efficacious in targeted radiotherapy when labelled with 90Y and/or 177Lu provided that there is no significant difference among these MIII radiometals.
To understand the contribution of the high uptake values in SRIF receptor-positive organs due to the different subtype affinities of [111In]DOTA-NOC, the uptake in these tissues was studied using different blocking agents, namely DTPA-TATE, an sstr2-specific ligand with IC50=3.9±1 nM, and InIII-DOTA-NOC, which has a high affinity to sstr2, sstr3 and sstr5. The higher blocking efficiency of InIII-DOTA-NOC in the adrenals, pancreas, stomach and pituitary may indicate that part of the radioligand uptake is due to the improved receptor subtype profile. These organs have previously been shown to express different receptor subtypes, at least at the mRNA level: sstr2: adrenals, pituitary and pancreas; sstr3: pituitary, pancreas and stomach; sstr5: adrenals, pituitary, pancreas and stomach [51].
In conclusion, we have developed a new radiopeptide based on somatostatin which promises to target a broader range of somatostatin receptors and concomitantly a larger spectrum of tumours. These preclinical data indicate that [111In]DOTA-NOC is superior to existing and well-studied radiolabelled somatostatin analogues. Indeed, the predictions from these preclinical studies have been confirmed in initial clinical studies in which excellent images of thyroid cancer patients have been obtained. We assume that [90Y]/[177Lu]DOTA-NOC will have similar favourable properties.
References
Behr TM, Béhé M, Becker W. Diagnostic applications of radiolabeled peptides in nuclear endocrinology. Q J Nucl Med 1999; 43:268–280.
Breeman WAP, de Jong M, Kwekkeboom DJ, et al. Somatostatin receptor-mediated imaging and therapy: basic science, current knowledge, limitations and future perspectives. Eur J Nucl Med 2001; 28:1421–1429.
Fischman A, Babich J, Strauss H. A ticket to ride: peptide radiopharmaceuticals. J Nucl Med 1993; 34:2253–2263.
Heppeler A, Froidevaux S, Eberle A, Maecke H. Receptor targeting for tumor localization and therapy with radiopeptides. Curr Med Chem 2000; 7:971–994.
Lamberts SW, Van der Lely AJ, De Herder WW, Hofland LJ. Octreotide. N Engl J Med 1996; 25:246–254.
Lister-James J, Moyer B, Dean T. Small peptides radiolabeled with99mTc. Q J Nucl Med 1996; 40:221–233.
Liu S, Edwards D.99mTc-labeled small peptides as diagnostic radiopharmaceuticals. Chem Rev 1999; 99:2235–2268.
Reubi JC. Neuropeptide receptors in health and disease: the molecular basis for in vivo imaging. J Nucl Med 1995; 36:1825–1835.
Thakur M. Radiolabelled peptides: now and the future. Nucl Med Comm 1995; 16:724–732.
Krenning EP, Kwekkeboom DJ, Bakker WH, et al. Somatostatin receptor scintigraphy with [111In-DTPA-d-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med 1993; 20:716–731.
Gibril F, Reynolds JC, Doppman JL, et al. Somatostatin receptor scintigraphy: its sensitivity compared with that of other imaging methods in detecting primary and metastatic gastrinomas—a prospective study. Ann Intern Med 1996; 125:24–26.
Blum JE, Handmaker H, Rinne NA. The utility of a somatostatin-type receptor binding peptide radiopharmaceutical (P829) in the evaluation of solitary pulmonary nodules. CHEST 1999; 115:224–232.
Virgolini I, Leimer M, Handmaker H, et al. Somatostatin receptor subtype specificity and in vivo binding of a novel tumor tracer,99mTc-P829. Cancer Res 1998; 58:1850–1859.
Lebtahi R, Le Cloirec J, Houzard C, et al. Detection of neuroendocrine tumors:99mTc-P829 scintigraphy compared with 111In-pentetreotide scintigraphy. J Nucl Med 2002; 43:889–895.
de Jong M, Bakker WH, Krenning EP, et al. Yttrium-90 and indium-111 labelling, receptor binding and biodistribution of [DOTA0,d-Phe1,Tyr3]octreotide, a promising somatostatin analogue for radionuclide therapy. Eur J Nucl Med 1997; 24:368–371.
Stolz B, Weckbecker G, Smith-Jones PM, Albert R, Raulf F, Bruns C. The somatostatin receptor-targeted radiotherapeutic [90Y-DPTA-dPhe1,Tyr3]octreotide (90Y-SMT 487) eradicates experimental rat pancreatic CA 20948 tumours. Eur J Nucl Med 1998; 25:668–674.
Otte A, Herrmann R, Heppeler A, et al. Yttrium-90-DOTATOC: first clinical results. Eur J Nucl Med 1999; 26:1439–1447.
Otte A, Mueller-Brand J, Dellas S, Nitzsche E, Herrmann R, Maecke H. Yttrium-90-labelled somatostatin-analogue for cancer treatment. Lancet 1998; 351:417–418.
Cremonesi M, Ferrari M, Zoboli S, et al. Biokinetics and dosimetry in patients administered with111In-DOTA-Tyr3-octreotide: implications for internal radiotherapy with 90Y-DOTATOC. Eur J Nucl Med 1999; 26:877–886.
Waldherr C, Pless M, Maecke H, et al. Tumor response and clinical benefit in neuroendocrine tumors after 7.4 GBq90Y-DOTATOC. J Nucl Med 2002; 43:610–616.
Paganelli G, Zoboli S, Cremonesi M, et al. Receptor-mediated radiotherapy with90Y-DOTA-Phe1-Tyr3-octreotide. Eur J Nucl Med 2001; 28:426–434.
Waldherr C, Pless M, Maecke H, Haldemann A, Mueller-Brand J. The clinical value of [90Y-DOTA]-d-Phe1-Tyr3-octreotide (90Y-DOTATOC) in the treatment of neuroendocrine tumours: a clinical phase II study. Ann Oncol 2001; 12:942–945.
de Jong M, Breeman WA, Bernard BF, et al. Tumor response after [90Y-DOTA0,Tyr3]octreotide radionuclide therapy in a transplantable rat tumor model is dependent on tumor size. J Nucl Med 2001; 42:1841–1846.
Reubi J, Schaer J, Waser B, et al. Affinity profiles for human somatostatin receptor sst1–sst5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur J Nucl Med 2000; 27:273–282.
Kwekkeboom DJ, Bakker WH, Kooij PP, et al. [177Lu-DOTA0,Tyr3]octreotate: comparison with [111DTPA0]octreotide in patients. Eur J Nucl Med 2001; 28:1319–1325.
Reubi JC, Waser B, Schaer J, Laissue JA. Somatostatin receptor sst1–sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur J Nucl Med 2001; 28:836–846.
Halmos G, Sun B, Schally AV, Hebert F, Nagy A. Human ovarian cancers express somatostatin receptors. J Clin Endocrinol Metab 2000; 85:3509–3512.
Reubi JC, Schaer J, Waser B, Mengod G. Expression and localization of somatostatin receptor sstr1, sstr2, and sstr3 messenger RNAs in primary human tumors using in situ hybridization. Cancer Res 1994; 54:3455–3459.
Forssell-Aronsson E, Nilsson O, Benjegard SA, et al.111In-DTPA-d-Phe1-octreotide binding and somatostatin receptor subtypes in thyroid tumors. J Nucl Med 2000; 41:636–642.
Raderer M, Pangerl T, Leimer M, et al. Expression of human somatostatin receptor subtype 3 in pancreatic cancer in vitro and in vivo. J Nat Cancer Inst 1998; 90:1666–1668.
Traub T, Petkov V, Ofluoglu S, et al.111In-DOTA-lanreotide scintigraphy in patients with tumors of the lung. J Nucl Med 2001; 42:1309–1315.
Heppeler A, Froidevaux S, Maecke HR, et al. Radiometal-labelled macrocyclic chelator-derivatised somatostatin analogue with superb tumour targeting properties and potential for receptor-mediated internal radiotherapy. Chem Eur J 1999; 5:1974–1981.
Atherton E, Sheppard R. Fluorenylmethoxycarbonyl-polyamide solid phase peptide synthesis. General principles and development. Oxford: Oxford Information Press, 1989.
Oberg K. Established clinical use of octreotide and lanreotide in oncology. Chemotherapy 2001; 47:40–53.
Chiti A, Fanti S, Savelli G, et al. Comparison of somatostatin receptor imaging, computed tomography and ultrasound in the clinical management of neuroendocrine gastro-entero-pancreatic tumours. Eur J Nucl Med 1998; 25:1396–1403.
Lebtahi R, Cadiot G, Sarda L, et al. Clinical impact of somatostatin receptor scintigraphy in the management of patients with neuroendocrine gastroenteropancreatic tumors. J Nucl Med 1997; 38:853–858.
Cadiot G, Bonnaud G, Lebtahi R, et al. Usefulness of somatostatin receptor scintigraphy in the management of patients with Zollinger-Ellison syndrome. Gut 1997; 41:107–114.
Termanini B, Gibril F, Reynolds JC, et al. Value of somatostatin receptor scintigraphy: a prospective study in gastrinoma of its effect on clinical management. Gastroenterology 1997; 112:335–347.
Gibril F, Doppman JL, Reynolds JC, et al. Bone metastases in patients with gastrinomas: a prospective study of bone scanning, somatostatin receptor scanning, and MRI in their detection, their frequency, location and effect of their detection on management. J Clin Oncol 1998; 16:1040–1053.
Krenning EP, de Jong M, Kooij PP, et al. Radiolabelled somatostatin analogue(s) for peptide receptor scintigraphy and radionuclide therapy. Ann Oncol 1999; 10 (Suppl 2):S23–S29.
Janson E, Eriksson B, Oberg K. Treatment with high dose [111In-DTPA-d-Phe1]-octreotide in patients with neuroendocrine tumors. Acta Oncol 1999; 38:373–377.
Valkema R, de Jong M, Bakker WH, et al. Phase 1 study of peptide receptor radionuclide therapy with [111In-DTPA0]-octreotide: the Rotterdam experience. Semin Nucl Med 2002; 32:110–123.
de Jong M, Breeman WA, Bernard BF, et al. [177Lu-DOTA0,Tyr3]Octreotate for somatostatin receptor-targeted radionuclide therapy. Int J Cancer 2001; 92:628–633.
Lewis J, Lewis M, Srinivasan A, Schmidt MA, Wang J, Anderson CJ. Comparison of four64Cu-labeled somatostatin analogues in vitro and in tumor-bearing rat model: evaluation of new derivatives for positron emission tomography imaging and targeted radiotherapy. J Med Chem 1998; 42:1341–1347.
Froidevaux S, Heppeler A, Eberle A, et al. Preclinical comparison in AR4-2J tumor bearing-mice of four radiolabeled 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-somatostatin analogs for tumor diagnosis and internal radiotherapy. Endocrinology 2000; 141:3304–3312.
Reubi JC, Eisenwiener KP, Rink H, Waser B, Maecke H. A new peptide somatostatin agonist with high affinity to all five somatostatin receptors. Eur J Pharmacol 2002; 456:45–49.
Smith-Jones PM, Bischof C, Leimer M, et al. DOTA-lanreotide: a novel somatostatin analog for tumor diagnosis and therapy. Endocrinology 1999; 140:5136–5148.
Maecke H, Scherer G, Heppeler A, Hennig M. Is In-111 an ideal surrogate for Y-90? If not why? Eur J Nucl Med 2001; 28:967.
Eisenwiener KP, Prata MIM, Buschmann I, et al. NODAGATOC, a new chelator-coupled somatostatin analogue labeled with [67/68Ga] and [111In] for SPECT, PET, and targeted therapeutic applications of somatostatin receptor (hsst2) expressing tumors. Bioconj Chem 2002; 13:530–541.
Koenig JA, Kaur R, Dodgeon I, Edwardson JM, Humphrey PPA. Fates of endocytosed somatostatin sst2 receptor and associated agonists. Biochem J 1998; 336:291–298.
Raulf F, Pérez J, Joyer D, Bruns C. Differential expression of five somatostatin receptor subtypes, sstr1-5, in the CNS and peripheral tissue. Digestion 1994; 55 (Suppl 3):46–53.
Acknowledgements
M. Ginj, D. Wild, J. Schmitt and H. Maecke acknowledge support from the Swiss National Science Foundation project No. 31-52969.97, BBW No. C00.0091 and BBT project 4668.1 EUS. The support provided by Novartis Pharma in respect of MS and NMR is gratefully acknowledged.
This work was performed within the COST B12 action.
Author information
Authors and Affiliations
Corresponding author
Additional information
Abbreviations of the common amino acids are in accordance with the recommendations of IUPAC-IUB [IUPAC-IUB Commission of Biochemical Nomenclature (CBN), Symbols for amino-acid derivatives and peptides, recommendations 1971. Eur J Biochem 1972; 27:201–207].
Rights and permissions
About this article
Cite this article
Wild, D., Schmitt, J.S., Ginj, M. et al. DOTA-NOC, a high-affinity ligand of somatostatin receptor subtypes 2, 3 and 5 for labelling with various radiometals. Eur J Nucl Med Mol Imaging 30, 1338–1347 (2003). https://doi.org/10.1007/s00259-003-1255-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-003-1255-5