Abstract
Purpose
The present paper reports a systematic study on the effect of bifunctional chelators (BFC) namely, NOTA, DOTA, and DTPA, on the radiochemical formulation, in vitro stability, and in vivo biological properties of 68Ga-labeled RGD peptide derivatives.
Methods
The three RGD conjugates namely, NOTA-Bn-E-[c(RGDfk)]2, DOTA-Bn-E-[c(RGDfk)]2, and DTPA-Bn-E-[c(RGDfk)]2 were radiolabeled with 68Ga and the radiolabeling was optimized with respect to the ligand amount, radiolabeling time, and temperature. Further, the 68Ga complexes were assessed for their in vitro and in vivo stabilities. The biodistribution studies of the three radiolabeled conjugates were carried out in C57BL/6 mice bearing melanoma tumor at 30 min and 1 h post-adimistration.
Results
NOTA-Bn-E-[c(RGDfk)]2 could be radiolabeled with 68Ga at room temperature while DOTA-Bn-E-[c(RGDfk)]2 and DTPA-Bn-E-[c(RGDfk)]2 were radiolabeled at high temperature. 68Ga-NOTA-Bn-E-[c(RGDfk)]2 was found to be the most kinetically rigid in in vitro stability assay. The uptake of the three radiolabeled peptide conjugates in melanoma tumor was comparable at 1 h post-administration (NOTA; DOTA; DTPA (% I.D./g):: 2.78 ± 0.38; 3.08 ± 1.1; 3.36 ± 0.49). However, the tumor/background ratio of 68Ga-NOTA-Bn-E-[c(RGDfk)]2 was the best amongst the three radiotracers. 68Ga-complexes of NOTA-Bn-E-[c(RGDfk)]2 and DOTA-Bn-E-[c(RGDfk)]2 showed excellent in vivo stability while 68Ga-DTPA-Bn-E-[c(RGDfk)]2 showed significant metabolic degradation.
Conclusion
These studies show that 68Ga-NOTA-Bn-E-[c(RGDfk)]2 would be the most appropriate 68Ga-labeled radiotracer and the most amenable for kit formulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tumor angiogenesis is an essential requirement for tumor growth and metastasis where integrin αvβ3 plays a major role [1,2,3,4,5]. The αvβ3 integrins act as a receptor for extracellular matrix proteins with the exposed arginine-glycine-aspartic acid (RGD) tripeptide sequence. They are over-expressed on large number of activated endothelial cells during angiogenesis in contrast to the resting endothelial cells. Also, αvβ3 integrins are overexpressed in some tumors cells like osteosarcomas, neuroblastomas, glioblastomas, melanomas, lung carcinomas and breast cancer and help in their growth and metastasis. Thus, imaging of αvβ3 integrins is considered as a useful tool in management of cancer [1,2,3,4,5].
In the last decade, a variety of RGD peptide based radiotracers have been developed for imaging αvβ3 integrins. In this context, both single photon emitters and positron emitting radionuclides have been explored and tagged with various RGD analogues [6,7,8,9,10,11]. Currently, use of positron emitting radionuclides like 18F, 68Ga, 64Cu, etc. has been growing clinically owing to the high resolution, greater sensitivity, and possibility of quantification by positron emission tomography (PET) [12]. Various 18F labeled RGD peptides have been extensively evaluated preclinically and clinically for PET imaging of αvβ3 integrins positive tumors [13, 14]. However, due to disadvantages like cyclotron based production of 18F as well as a radiosynthetic module required for isolating clinical grade product, which increases the preparation cost of the final product, more and more attention is now being given to 68Ga based RGD radiotracers for widespread clinical use.
This is due to advantages like availability of 68Ga from the 68Ge/68Ga generator system which can facilitate the growth of PET imaging in areas that are remote from cyclotron facilities [15]. Its well developed coordination chemistry allows for development of 68Ga-radiotracers with high yield and specific activity which obviates the need of expensive modules for post labeling purification processes [16,17,18]. Also, the short half life of 68Ga (t1/2 = 68 min) is ideal for imaging of small molecules and peptides that clear quickly from the background tissues.
There are numerous reports on the evaluation of 68Ga-labeled RGD peptides in animal models as well as in human patients wherein the RGD-motif has been conjugated to either DOTA or NOTA/NODAGA chelators [19,20,21,22,23,24,25,26,27]. Nevertheless, a detailed systematic evaluation of 68Ga-labeled RGD peptide derivative coupled to commonly used bifunctional chelators, which would clearly demonstrate the specific role of BFCs on the radiochemistry, in vitro stability, pharmacokinetics, tumor targeting properties, and metabolic stability in suitable animal model, is an interesting proposition. Toward this, in the present paper, we report a detailed and systemic comparison of the aforementioned properties of 68Ga-complexes of dimeric cyclic RGD peptide E[c(RGDfK)]2 (E = glutamic acid, R = arginine, G = glycine, D = aspartic acid, f = D-phenylalanine, K = lysine) conjugated with different cyclic and acyclic BFCs namely, p-SCN-Bn-NOTA [S-2-(4-isothiocyanatobenzyl)-1,4,7-triazacyclononane-1,4,7-triacetic acid], p-SCN-Bn-DOTA [S-2-(4- isothiocyanatobenzyl)-1,4,7,10-tetraazacyclododecane-1,4,7,10- tetraacetic acid], and p-SCN-Bn-DTPA [S-2-(4-isothiocyanatobenzyl)-diethylenetriamine pentaacetic acid]. Such a systematic evaluation would be helpful in choosing the most appropriate BFC-(RGD)2 peptide conjugates for the development of 68Ga-labeled radiotracers for routine PET imaging of tumor angiogenesis in clinical settings.
Materials
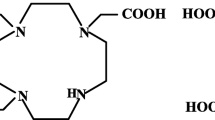
The RGD peptide conjugates namely NOTA-Bn-E-[c(RGDfK)]2 (NOTA-Bn-(RGD)2), DOTA-Bn-E-[c(RGDfK)]2 (DOTA-Bn-(RGD)2), and DTPA-Bn-E-[c(RGDfK)]2 (DTPA-Bn-(RGD)2) (Fig. 1a–c) were custom synthesized by ABX Advance Biomedical Compounds, Radeber, Germany. Suprap® hydrochloric acid (99.999% pure) was procured from Sigma-Aldrich, India. MilliQ water (resistivity >18.2 MΩ) was used in all the wet chemistry studies. All other chemicals used were of analytical grade and procured from Sigma-Aldrich, India. Gallium-68 was eluted in 0.6 M HCl from a 925 MBq (25 mCi) 68Ge/68Ga radionuclide generator obtained from iThemba Labs, South Africa.
High performance liquid chromatography (HPLC) analyses were carried out on a JASCO PU 2080 Plus dual pump HPLC system (JASCO, Japan) coupled with a JASCO 2075 Plus tunable absorption detector and Gina Star radioactivity detector system (Raytest, Germany). C18 reversed phase HiQ Sil (5 μm, 4 × 250 mm) column was used as the stationary phase, while a mixture of acetonitrile and water (both containing 0.1% TFA) was used as the mobile phase. A well-type NaI(Tl) detector (Mucha, Raytest, Germany) was used for radioactivity measurements during all other radiochemical studies. Radioactivity measurements during animal biodistribution studies were carried out using a flat-type NaI(Tl) radioactivity detector (Harshaw, UK).
Experimental
Radiolabeling of BFC-(RGD)2 Conjugates with 68Ga
The 68Ga radiolabeling procedure followed for the three BFC-(RGD)2 conjugates (Fig. 1a–c) was identical. Typically, BFC-(RGD)2 conjugate (1 μg/μL) in MilliQ water was mixed with 2 M sodium acetate solution (450 μL, pH ~7.5). Further, 68GaCl3 (1 mL, 185 MBq/5 mCi) in 0.6 N HCl was added to it and the reaction mixture was then incubated at room temperature (25 °C) or in a heated water bath (90 °C). The final volume was ~1.5 mL and the final pH was ~3.5. The radiolabeling parameters were optimized by varying the amount of BFC-(RGD)2 as well as the incubation time and temperature in order to maximize the radiochemical yield. All subsequent reactions were done in the respective optimized conditions for each BFC-(RGD)2 conjugate.
Determination of the Radiochemical Yield and Purity
The radiochemical yield of 68Ga-BFC-(RGD)2 was determined by paper chromatography (PC) as well as by high performance liquid chromatography (HPLC) techniques:
Paper Chromatography
Paper chromatography was performed using Whatman No. 1 chromatography paper. 5 μL (600 KBq/ 17 μCi) of test solution was applied at 1.5 cm from the lower end of the strip and it was developed in 0.01 M Na citrate solution (pH = 5). The strips were then dried and cut into segments of 1 cm each and the activity associated with each segment was measured in NaI(Tl) detector.
HPLC Technique
HPLC was carried out following gradient elution technique using a dual pump HPLC system and C18 reversed phase HiQ Sil (5 μm, 4 × 250 mm) column. Water [A] and acetonitrile [B] with 0.1% trifluoroacetic acid were used as the mobile phase and the following gradient elution method was used for separation: 0–4 min 95% A, 4–15 min 95% A to 5% A, 15–20 min 5% A, 20–25 min 5% A to 95% A, 25–30 min 95% A. All the solvents were of HPLC grade, degassed and filtered prior to use. The flow rate was maintained at 1 mL/min. About 10 μL (~1.20 MBq/30 μCi) of the test solution was injected into the HPLC. The elution was monitored both by UV and by radioactivity signal.
Determination of Partition Coefficient (Log P) of 68Ga-BFC-(RGD)2 Radiotracers
For determining log P, an aliquot of 68Ga-BFC-(RGD)2 complex (100 μL, 12.3 MBq/300 μCi) was mixed with water (0.9 mL) and octanol (1 mL) on a vortex mixer and then centrifuged to effect the separation of the two layers. Equal aliquots from both layers were counted in NaI(Tl) detector. Partition coefficient (log P) was expressed as the logarithm of the ratio of the counts from n-octanol versus that of the aqueous layer. Further, n-octanol layer was repartitioned until a consistent partition coefficient was obtained.
In Vitro Stability of 68Ga-BFC-(RGD)2 Radiotracers
Stability in EDTA Solution
In order to ascertain the stability of the 68Ga-BFC-(RGD)2 under EDTA challenge, an aliquot of each complex (50 μL, 6.2 MBq/0.16 mCi) was incubated with 5 mM EDTA solution (450 μL) for different time intervals. Aliquots were withdrawn at intervals of 30 min, 60 min, 90 min, and 120 min. and analyzed by HPLC to assess the stability of the complex.
Stability in Human Serum
The in vitro stability of the 68Ga-BFC-(RGD)2 radiotracers in human serum was determined by incubating each complex (50 μL, 5.6 MBq/0.15 mCi) with human serum (450 μL) at 37 °C for different time intervals. Aliquots were withdrawn at intervals of 30 min, 60 min, 90 min, and 120 min, ethanol was added to precipitate the serum proteins, centrifuged and the supernatant was analyzed by paper chromatography using 0.01 M sodium citrate solution (pH = 5) to determine the stability of the complex. In 0.01 M sodium citrate solution, 68Ga-complex (Rf = 0.0) remained at the point of spot while free 68Ga3+ moved with the solvent front (Rf = 0.9–1.0).
In Vivo Evaluation 68Ga-BFC-(RGD)2 Radiotracers
All in vivo procedures performed herein were in strict compliance with the approved protocols of Institutional Animal Ethics Committee of Bhabha Atomic Research Centre, India.
Biodistribution Studies in Animal Model
Biological efficacy of the 68Ga-BFC-(RGD)2 radiotracers was evaluated in female C57BL/6 mice (age: 6–8 week, weight: 20–25 g weight) bearing melanoma tumors. Melanoma B16-F10 cell line (ATCC-CRL-6475TM) used for growing the tumors, was purchased from National Center for Cell Science (India). The tumors were developed by injecting ~1 × 106 B16-F10 cells, suspended in 200 μL of PBS, subcutaneously into the right thigh of each C57BL/6 mice weighing 20–25 g. The animals were reared and kept in laboratory animal facility of our institute under standard management practice for about 2 weeks. The animal experiments were done when visible tumors were observed and a tumor mass of 0.2–0.4 g was attained. The radiolabeled preparation (100 μL, 370 KBq/10 μCi) was administered intravenously through the tail vein of each animal. Individual sets of animals (n = 4) were utilized for studying the bio-distribution at different time points (30 min, 60 min). The animals were sacrificed by carbon dioxide asphyxiation immediately at the end of the respective time point and the relevant organs and tissue were excised for measurement of associated activity. The organs were weighed and the activity associated with each of them was measured in a flat-bed type NaI(Tl) counter with suitable energy window for 68Ga. For the purpose of uniformity, the activity retained in each organ/tissue was expressed as a percent value of the injected dose per gram (% ID/g). Activity associated with the excreta (urine + feces) was determined by counting the cage paper which was expressed as percent value of the injected dose (% ID).
Assay of Metabolic Stability
Metabolic stability of the radiotracers was analyzed by the following method. 68Ga-BFC-(RGD)2 radiotracers (100 μL, 370 KBq/10 μCi) were injected in normal Swiss mice (n = 2) intravenously through the lateral tail vein of each animal. The urine sample of the animal was collected 1 h post injection. Acetonitrile was added to the urine sample to precipitate the proteins. The sample was then centrifuged and the supernatant was analyzed using HPLC.
Results
Radiolabeling of BFC-(RGD)2 Conjugates with 68Ga
The radiolabeling yields of the three 68Ga-BFC-(RGD)2 tracers namely, 68Ga-NOTA-Bn-(RGD)2, 68Ga-DOTA-Bn-(RGD)2, and 68Ga-DTPA-Bn-(RGD)2 were determined by PC and HPLC technique. In paper chromatography, it was observed that free 68Ga moved to the solvent front (Rf = 0.9–1) while 68Ga-complex remained at the point of spot (Rf = 0.0). A typical PC pattern is depicted in Fig. 2. The radio-HPLC profiles are given in Fig. 3. It was observed that 68Ga-NOTA-Bn-(RGD)2, 68Ga-DOTA-Bn-(RGD)2, and 68Ga-DTPA-Bn-(RGD)2 exhibited retention times of 15.3 min, 15.6 min, and 16.1 min, respectively, while free 68GaCl3 eluted before 4 min.
With an aim of preparing 68Ga-BFC-(RGD)2 conjugates with maximum yield, the radiolabeling experiments were carried out by varying different reaction parameters, such as ligand concentration, incubation temperature, and reaction time. Figure 4 illustrates the effect of ligand concentration on the radiolabeling yield of 68Ga-BFC-(RGD)2 radiotracers at room temperature (25 °C) and at 90 °C, respectively, when the reaction mixtures were incubated for 10 min. It was observed that only 10 μg of NOTA-Bn-(RGD)2 was sufficient for ≥98% radiolabeling yield at room temperature. In the case of DTPA-Bn-(RGD)2, radiolabeling yield ≥98% could be achieved using 10 μg of peptide conjugate when the reaction mixture was heated at 90 °C for 10 min. On the other hand, in order to achieve >95% yield with DOTA-Bn-(RGD)2, 50 μg of peptide conjugate was required and the reaction mixture needed to be heated at 90 °C for 10 min. In order to have a comparative assessment of the kinetics of formation of 68Ga-complexes, the radiochemical yields were determined at different time intervals on incubation of reaction mixtures at room temperature as well as at elevated temperature. The results are illustrated in Table 1.
The table shows that when incubated at room temperature, in the case of NOTA-Bn-(RGD)2, ≥95% yield could be obtained within 5 min of incubation. However, in the case of DTPA-Bn-(RGD)2 and DOTA-Bn-(RGD)2 a maximum yield of ~80–85% could be achieved when the reaction mixtures were incubated for at least 20 min at room temperature. When the reaction mixtures were incubated at 90 °C, the kinetics of 68Ga complexation with DTPA-Bn-(RGD)2 and DOTA-Bn-(RGD)2 improved significantly. Overall, 68Ga-NOTA-Bn-(RGD)2 exhibited the most favorable formation kinetics followed by 68Ga-DTPA-Bn-(RGD)2.
The optimized protocols for the syntheses of 68Ga-BFC-(RGD)2 conjugates along with their maximum specific activity achieved are given in Table 2.
Log P of 68Ga-BFC-(RGD)2
The log P values for 68Ga-NOTA-Bn-(RGD)2, 68Ga-DOTA-Bn-(RGD)2, and 68Ga-DTPA-Bn-(RGD)2 were −0.3 ± 0.05, −0.39 ± 0.04, and −0.58 ± 0.02, respectively. This shows that 68Ga-DTPA-(RGD)2 has the most hydrophilic nature amongst the three radiotracers. The log P values of the radiotracers are given in Table 2.
In Vitro Stability Studies
The radiochemical purities of 68Ga-BFC-(RGD)2 radiotracers when incubated in EDTA solution and human serum are given in Fig. 5, respectively. No significant transchelation was observed when the three tracers were incubated in excess EDTA solution and their radiochemical purities were ≥90% upto 120 min. The radiochemical purities of 68Ga-NOTA-Bn-(RGD)2 and 68Ga-DOTA-Bn-(RGD)2 were found to be ≥95% upto 120 min in human serum. However, in the case of 68Ga-DTPA-Bn-(RGD)2, a gradual decrease in its radiochemical purity with time was observed.
In Vivo Evaluation 68Ga-BFC-(RGD)2 Radiotracers
The results of biodistribution studies of the three 68Ga-BFC-(RGD)2 radiotracers in C57BL/6 mice bearing melanoma tumors are given in Table 3. Amongst the three radiotracers, 68Ga-DOTA-Bn-(RGD)2 exhibited the highest tumor uptake of 5.08 ± 0.05% ID/g at 30 min p.i., which decreased to 3.08 ± 1.10% ID/g at 1 h p.i. The liver uptake of 68Ga-DOTA-Bn-(RGD)2 was also high at both 30 min (4.47 ± 1.02%ID/g) and 1 h p.i. (3.59 ± 0.18%ID/g). The melanoma tumor uptake of 68Ga-NOTA-Bn-(RGD)2 was high at 30 min p.i. (4.50 ± 0.18% ID/g), but it also decreased at 1 h p.i. (2.78 ± 0.38% ID/g). On the other hand, the highest tumor uptake observed in the case of 68Ga-DTPA-Bn-(RGD)2 was considerably lower compared to the other two radiotracers (3.51 ± 0.69% ID/g at 30 min p.i), which nearly retained upto 1 h p.i. (3.36 ± 0.49% ID/g). A comparison of the tumor/background ratios of the three radiotracers is illustrated in Fig. 6. It is evident from the figure that, in general, 68Ga-NOTA-Bn-(RGD)2 radiotracer exhibited the best tumor/background ratio and hence is expected to generate the best target/background contrast in clinical context. All three radiotracers predominantly exhibited urinary excretion.
In order to ascertain the in vivo stability of 68Ga-BFC-(RGD)2 radiotracers, the urine samples of Swiss mice injected with the respective radiotracers were analyzed in HPLC 1 h p.i.. The radio chromatograms of the urine samples of the three tracers are given in Fig. 7. From the figure it can be seen that both 68Ga-NOTA-Bn-(RGD)2 and 68Ga-DOTA-Bn-(RGD)2 radiotracers were stable in vivo as only one peak corresponding to the tracers at 15.3 min and 15.6 min respectively, were observed. However, in the case of 68Ga-DTPA-Bn-(RGD)2, a small metabolite peak below 5 min (8%) was observed along with radiotracer peak at 16.07 min (92%). This shows a decrease in stability of 68Ga-DTPA-Bn-(RGD)2 in vivo.
Discussion
Tumor angiogenesis is one of the most widely studied areas for cancer imaging and treatment and RGD-based PET imaging plays a pivotal role in this aspect. The advantage of 68Ga as a PET radionuclide lies in its easy availability through a 68Ge/68Ga generator which obviates the need of an in-house cyclotron. Another advantage is the straightforward labeling strategies for 68Ga which assist in synthetic automation. There are a large number of reports on 68Ga labeled RGD peptide derivatives for PET imaging of tumors over-expressing αvβ3 integrins [19,20,21,22,23,24,25,26,27]. The main objective of the present study was to demonstrate the influence of the chelation of 68Ga on the pharmacokinetic properties of 68Ga-labeled tumor targeting peptide conjugates by a systematic comparative evaluation of dimeric RGD peptide analogues conjugated to three different chelators namely, NOTA, DOTA, and DTPA. RGD dimers are known to yield better tumor uptake results as compared to their monomeric analogues. This is because of an increase in local RGD concentration, i.e., binding of one RGD motif increases the ‘local concentration’ of the second RGD motif in the vicinity of integrin αvβ3. Further, on increasing the multiplicity of the peptide (trimer, tetramer, etc.) the uptake of the analogue significantly increases in kidney, liver, lungs, and spleen. On account of these literature reports, dimeric RGD analogue was used in the present study.
The three RGD-conjugates (Fig. 1a–c) were radiolabeled with 68Ga and the radiolabeling conditions were optimized with respect to the peptide amount, reaction temperature, and incubation time. The optimization process is important to facilitate development of kits which can further be translated to clinical settings for routine preparation of 68Ga based radiotracers for imaging. Since the half-life of 68Ga is short (t1/2 = 68 min), a fast radiolabeling without the need of post labeling purification would be desirable as it would ensure a minimum loss of imaging agent to decay. Also, a final product with high specific activity would be advantageous toward receptor based imaging since an excess cold peptide might compete for the receptor binding sites effectively reducing target uptake. It was observed that NOTA-conjugated RGD derivative could be radiolabeled with 68Ga at room temperature with high radiolabeling yield (≥ 98%) and high specific activity (~ 20.3 GBq/μmol). The DTPA-conjugated analogue could also be synthesized at high specific activity (~ 22.6 GBq/μmol) but at a higher temperature. On the other hand, DOTA-conjugated peptide could be radiolabeled in adequately high yield at high temperature as well as with higher peptide amount, so that the specific activity of the radiotracer was significantly lower (~4.6 GBq/μmol). Moreover, amongst the peptide conjugates studied, the radiolabeling conditions of NOTA-Bn-(RGD)2 are most suitable for kit preparation in clinical settings.
Although the three radiotracers were prepared with high radiochemical purity (RCP), it is important that their RCP is maintained when they are injected into the blood stream. In the blood stream, the concentration of the tracer would be very low as compared to other metal ions which can favor appreciable dissociation of 68Ga3+ from the radiometal-BFC complex. Also, due to the presence of various biomolecules in the blood stream which may have affinity for 68Ga, significant transchelation reactions may take place. These dissociation and transchelation reactions of 68Ga3+ may lead to unwanted accumulation of radioactivity in non-target organs. Thus, the stability of the three radiotracers was examined in vitro by incubating them in human serum at 37 °C up to 2 h. No significant degradation was observed in the case of 68Ga labeled NOTA-Bn-(RGD)2 and DOTA-Bn-(RGD)2 tracers. This shows that both the radiotracers are practically unaffected by the presence of metal ions like Fe, Cu, Ca, and Zn and proteins like transferrin present in human serum. However, 10% degradation was observed when 68Ga-DTPA-Bn-(RGD)2 was incubated in human serum. This might be because of the higher kinetic lability of the 68Ga-chelate with acyclic chelator DTPA compared to those with macrocyclic chelators DOTA and NOTA [27, 28].
The log P values of the three radiotracers in water/octanol system suggest that all three radiotracers are hydrophilic in nature. Owing to their hydrophilic nature, the biodistribution studies showed clearance of the radiotracers mainly via the kidneys. In the case of 68Ga-DOTA-Bn-(RGD)2, a high tumor uptake was observed at 30 min p.i. However, significant accumulation of the radiotracer was also observed in non target organs which led to lower tumor/background ratios. An improvement in tumor/background ratio was observed at 1 h p.i. because of fast clearance of activity from the non target organs. The uptake of 68Ga-DOTA-Bn-(RGD)2 in liver was highest amongst the three radiotracers at both 30 min and 1 h p.i. These results were in tandem with that reported for other Ga labeled DOTA-RGD based compounds. (66Ga-DOTA-E[c(RGDfK)]2 30 min: 5–6% ID/g; 1 h: 3–4% ID/g).23
Although the uptake in tumor for 68Ga-NOTA-Bn-(RGD)2 was slightly lower as compared to 68Ga-DOTA-Bn-(RGD)2, the low uptake and fast clearance of radioactivity from non target organs like blood, liver, intestine, and lungs led to its high tumor/background contrast. This is a very desirable characteristic for a PET-radiotracer tumor imaging. In the case of 68Ga-DTPA-Bn-(RGD)2, the uptake in melanoma tumor was lower at 30 min p.i. and comparable at 1 h p.i. with respect to the other two radiotracers. On analysis of urine samples of animals injected with 68Ga-DTPA-Bn-(RGD)2, 7–8% degradation of the radiotracer was observed at in vivo within 1 h p.i. The low in vitro and in vivo stability of 68Ga-DTPA-Bn-(RGD)2 can be attributed to the acyclic nature of DTPA ligand which forms kinetically labile complexes [17, 28].
Conclusion
In summary, we have carried out a systematic comparative evaluation of three 68Ga-labeled RGD conjugates namely, NOTA-Bn-(RGD)2, DOTA-Bn-(RGD)2, and DTPA-Bn-(RGD)2. It was found that 68Ga-NOTA-Bn-(RGD)2 could be efficiently radiolabeled with 68Ga at room temperature with high radiochemical purity, specific activity, and in vitro and in vivo stability. Its preliminary biodistribution studies carried out in C57BL/6 mice indicated high tumor/background ratio along with clearance of the activity via the renal pathway. 68Ga-DTPA-(RGD)2 was also radiolabeled with high purity and high specific activity but its in vitro and in vivo stability was low. In the case of 68Ga-DOTA-Bn-(RGD)2, though the tumor uptake of the radiotracer was high, its tumor/background ratio was comparatively lower due to high accumulation of activity in non target organs. These studies indicate that 68Ga-NOTA-Bn-(RGD)2 would be the most suitable choice for further investigations toward the development of 68Ga based radiotracers for imaging tumor angiogenesis in clinical settings.
References
Backer MV, Backer JM. Imaging key biomarkers of tumor angiogenesis. Theranostics. 2012;2:502–15.
Cai W, Chen X. Multimodality molecular imaging of tumor angiogenesis. J Nucl Med. 2008;49:113S–28S.
Chakravarty R, Chakraborty S, Dash A. Molecular imaging of breast cancer: role of RGD peptides. Mini-Rev Med Chem. 2015;15:1073–94.
Danhier F, Le Breton A, Preat V. RGD-based strategies to target alpha(v) beta(3) integrin in cancer therapy and diagnosis. Mol Pharm. 2012;9:2961–73.
Weis SM, Cheresh DA. New approaches to image angiogenesis. Nat Med. 2011;17:1359–70.
Cai H, Conti PS. RGD-based PET tracers for imaging receptor integrin alphav beta3 expression. J Labelled Comp Radiopharm. 2013;56:264–79.
Haubner R, Maschauer S, Prante O. PET radiopharmaceuticals for imaging integrin expression: tracers in clinical studies and recent developments. Bio Med Res Int. 2014;871609:1–17.
Liu S. Radiolabeled cyclic RGD peptides as integrin αvβ3-targeted radiotracers: maximizing binding affinity via bivalency. Bioconjug Chem. 2009;20:2199–213.
Liu S. Radiolabeled multimeric cyclic RGD peptides as integrin αvβ3 targeted radiotracers for tumor imaging. Mol Pharm. 2006;3:472–87.
Niu G, Chen X. PET imaging of angiogenesis. PET Clin. 2009;4:17–38.
Tateishi U, Oka T, Inoue T. Radiolabeled RGD peptides as integrin αvβ3-targeted PET tracers. Curr Med Chem. 2012;19:3301–9.
Ziegler SI. Positron emission tomography: principles, technology, and recent developments. Nucl Phys A. 2005;752:679–87.
Iagaru A, Mosci C, Shen B, Chin FT, Mittra E, Telli ML, et al. 18F-FPPRGD2 PET/CT: pilot phase evaluation of breast cancer patients. Radiology. 2014;273:549–59.
Kenny LM, Coombe RC, Oulie I, Contractor KB, Miller M, Spinks TJ, et al. Phase I trial of the positron-emitting Arg-Gly-Asp (RGD) peptide radioligand 18F-AH111585 in breast cancer patients. J Nucl Med. 2008;49:879–86.
Röesch F, Riss PJ. The renaissance of the 68Ge/68Ga radionuclide generator initiates new developments in 68Ga radiopharmaceutical chemistry. Curr Top Med Chem. 2010;10:1633–68.
Goodwin DA, Ransone CM, Diamanti CI, McTigue M. Rapid synthesis and quality control of 68Ga-labeled chelates for clinical use. Nucl Med Biol. 1994;21:897–9.
Prata MI, Santos AC, Geraldes CF, De Lima JJ. Structural and in vivo studies of metal chelates of Ga(III) relevant to biomedical imaging. J Inorg Biochem. 2000;79:359–63.
Velikyan I, Maeck H, Langstrom B. Convenient preparation of 68Ga based PET radiopharmaceuticals at room temperature. Bioconjug Chem 2008; 19:569–573.
Chakraborty S, Chakravarty R, Vatsa R, Bhusari P, Sarma HD, Shukla J, et al. Toward realization of ‘mix-and-use’ approach in 68Ga radiopharmacy: preparation, evaluation and preliminary clinical utilization of 68Ga-labeled NODAGA-coupled RGD peptide derivative. Nucl Med Biol. 2016;43:116–23.
Dijkgraaf I, Yim CB, Franssen GM, Schuit RC, Luurtsema G, Liu S, et al. PET imaging of αvβ3 integrin expression in tumours with 68Ga-labelled mono-, di- and tetrameric RGD peptides. Eur J Nucl Med Mol Imaging. 2011;38:128–37.
Knetsch PA, Petrik M, Griessinger CM, Rangger C, Fani M, Kesenheimer C, et al. [68Ga]NODAGA-RGD for imaging alphavbeta3 integrin expression. Eur J Nucl Med Mol Imaging. 2011;38:1303–12.
Li ZB, Chen K, Chen X. 68Ga-labeled multimeric RGD peptides for microPET imaging of integrin alpha(v)beta (3) expression. Eur J Nucl Med Mol Imaging. 2008;35:1100–8.
Lopez-Rodriguez V, Gaspar-Carcamo RE, Pedraza-Lopez M, Rojas-Calderon EL, Arteaga de Murphy C, Ferro-Flores G, et al. Preparation and preclinical evaluation of 66Ga-DOTA-E(c(RGDfK))2 as a potential theranostic radiopharmaceutical. Nucl Med Biol. 2015;42:109–14.
Notni J, Pohle K, Wester HJ. Comparative gallium-68 labeling of TRAP-, NOTA-, and DOTA-peptides: practical consequences for the future of gallium-68-PET. EJNMMI Res. 2012;2:28.
Oxboel J, Brandt-Larsen M, Schjoeth-Eskesen C, Myschetzky R, El-Ali HH, Madsen J, et al. Comparison of two new angiogenesis PET tracers 68Ga-NODAGA-E[c(RGDyK)]2 and 64Cu-NODAGA-E[c(RGDyK)]2; in vivo imaging studies in human xenograft tumors. Nucl Med Biol. 2014;41:259–67.
Pohle K, Notni J, Bussemer J, Kessler H, Schwaiger M, Beer AJ. 68Ga-NODAGA-RGD is a suitable substitute for 18F-Galacto-RGD and can be produced with high specific activity in a cGMP/GRP compliant automated process. Nucl Med Biol. 2012;39:777–84.
Ferreira CL, Donald TYY, Mandel D, Gill KR, Boros E, Wong MQ, et al. 68Ga small peptide imaging: comparison of NOTA and PCTA. Bioconjug Chem. 2012;23:2239–46.
Chakravarty R, Chakraborty S, Dash A, Pillai MRA. Detailed evaluation on the effect of metal ion impurities on complexation of generator eluted 68Ga with different bifunctional chelators. Nucl Med Biol. 2013;40:197–205.
Acknowledgments
The authors are thankful to Dr. Aruna Korde, Head, Radiopharmaceutical Evaluation Section, Radiopharmaceuticals Division, BARC for providing access to the 68Ge/68Ga generator. Thanks are also due to Dr. B.S. Tomar, Director, Radiochemistry & Isotope Group, BARC for his support and encouragement.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
Akanksha Jain, Sudipta Chakraborty, Haladhar Dev Sarma and Ashutosh Dash, declare that they have no conflict of interest financial, scientific or otherwise in the publication of this article. Research at the Bhabha Atomic Research Centre (BARC) is part of the ongoing activities of the Department of Atomic Energy, India and is fully supported by government funding.
Ethical Approval
All procedures performed in studies involving animals were in strict compliance with the approved protocols of Institutional Animal Ethics Committee of BARC, India.
Informed Consent
The institutional review board of our institute, BARC, approved this retrospective study, and the requirement to obtain informed consent was waived.
Rights and permissions
About this article
Cite this article
Jain, A., Chakraborty, S., Sarma, H.D. et al. A Systematic Comparative Evaluation of 68Ga-Labeled RGD Peptides Conjugated with Different Chelators. Nucl Med Mol Imaging 52, 125–134 (2018). https://doi.org/10.1007/s13139-017-0499-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-017-0499-0