Abstract
A specialized ethylamine-bridged β-cyclodextrins (EB β-CD) was synthetized. EB β-CD’s adsorption properties of thorium ions were performed. Experimental results show that the adsorption of thorium using the EB β-CD can achieve equilibrium within 60 min at room temperature under the condition of pH 4, the adsorbance is 10.49 mg g−1. The results of SEM and BET nitrogen adsorption show that, EB β-CD is typical mesoporous material. According to pseudo-second-order kinetics, the adsorption of Th(IV) indicating the influence of textural properties of EB β-CD on the rate of adsorption. The thermodynamic parameters obtained showed that the adsorption process is exothermic, spontaneous process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the last years, a variety of technologies have been used for removal and recovery of thorium present in water, nuclear fuel effluents, and other sources in view of the potential environmental health threat and an nonrenewable resource of nuclear energy. So their gathering and separation from waste sources become a very significant task. A lot of work are being made to develop the processes for extracting thorium [1–5].
Recently, the use of organic materials as sorbents for removal of radioactive heavy metals in sorption processes has been used extensively. Sadeek et al. [6] reported chelating resin containing pentaethylenehexamine was used for adsorption of thorium(IV) as a functional group. Limin Zhou et al. [7] researched competitive adsorption of uranium(VI) and thorium(IV) ions from aqueous solution using triphosphate-crosslinked magnetic chitosan resins. Ji et al. [8] studied the adsorption of Th(IV) using surface modified dibenzoylmethane molecular imprinted polymer. These materials have lots of advantages, such as: good chemical stability, renewability, many possibilities to functionalization.
It is well-grounded that linking two cyclodextrin moieties with a short linker group greatly enhances the molecular binding ability of the original cyclodextrin through the cooperative binding of one guest molecule in the closely located two cyclodextrin cavities [9–13]. Hence, numerous bridged cyclodextrins with different structural materials have been synthesized, and applied to a variety of areas of science and technology such as molecular receptors [14, 15], electrochemical sensors [16], drug carriers [17] and monomer of polymer [18] in the last years. But the study of bridged β-cyclodextrins applied for recovery of thorium in waste warter has not been extensively investigated.
This paper described the synthesis method of a special bridged β-cyclodextrins(EB β-CD), studied on EB β-CD adsorption properties of thorium ions based on various experimental parameters (pH, amount of sorbent, contact time, temperature, thorium ion initial concentration), and analyzed the specific surface area and pore size of the synthetic EB β-CD by SEM and BET nitrogen adsorption, The experiment results showed that EB β-CD could effectively adsorb the Th(IV) in a high adsorbing capacity.
Material
β-cyclodextrin (biochemical reagent), Th(NO3)4·4H2O, 2-chloro ethylamine hydrochloride, P-paratoluensulfonyl chloride, N, N-dimethyl formamide were purchased from Shanghai Aladdin reagent co., LTD. Methyl alcohol and acetone, etc. were analytical reagent and used further purification. All the solutions used in this work was distilled water.
FTIR (Shimadzu, Japan), UV-spectrophotometer (Shanghai, China), Varian-400 High Resolution Nuclear Magnetic Resonance Spectrometer (Varian, America), NOVA2200e Multipoint Nitrogen Adsorption Instrument (Quantachrome, America), XL30 Scanning Electron Microscope (Philips, Netherlands), EA2400IICHNS/O Elemental Analyzer (PE, America).
Experimental
Preparetion of EB β-CD
Synthesis of mono-6-O-aminoethyl-β-cyclodextrin (6-EA-β-CD)
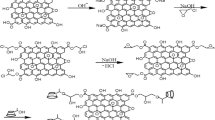
β-Cyclodextrin (1) was first soaked in aqueous solution of 2-chloroethylamine (20 %) (on weight of β-CD) for 30 min, and dried at 65 °C till solvent (water) was evaporated. The product was immersed in NaOH solution (2.5 mol L−1) for 45 min at temperature 70 °C. The 6-EA-β-CD (2) was obtained by precipitated in methyl alcohol. The 6-EA-β-CD (2) was washed several times and dried at 50 °C for 5 h (the yield is 46 %). The synthetic route is showed in the Fig. 1.
Synthesis of ethylamine-bridged β-cyclodextrins(EB β-CD)
Ethylamine-bridged β-cyclodextrins(EB β-CD) was synthetized using mono-6-O-(p-toluenesulfonyl)-β-cyclodextrin(3) and the 6-EA-β-CD (2) with the mole ratio of 2:1 in N,N-dimethyl formamide at temperature 80 °C for 72 h, followed by being poured into acetone, obtained the crude EB β-CD (4) as a precipitate. The crude product was purified on a column of Sephadex G-25 [19] with water as the eluent to give pure (4) in 27.6 % yield (on weight of 6-EA-β-CD). The synthetic route is showed in the Fig. 2.
Adsorption studies
Studies on the Th(IV) adsorption behavior were performed in batch experiments using 50 mL Erlenmeyer flasks. The effect of pH, amount of sorbent, equilibrium time and Th(IV) ion initial concentration were examined. Equilibrium studies were conducted within the amount of sorbent range of 10–30 mg, temperature range of 293–338 K, time range of 15–120 min and the metal ion concentration range of 5–30 mg L−1. The pH value of the different solutions was adjusted and measured (using buffered solution) accurately using a digital pH-meter of the type PHS-3C (Shanghai, China) within an error of ±0.1. 20 mg of the EB β-CD were added into a 50 mL Erlenmeyer flasks along with 25 mg L−1 Th(IV) at a given pH. The flasks were shaken using thermostatic water bath oscillators SHY-2A (Changsha, China) for specified durations at room temperature. After equilibration, the remanent concentration of the metal ion was determined by UV-spectrophotometer and adsorption amount Q (mg g−1) of adsorbed Th(IV) ions was calculated according to formula:
where Q is the adsorption quantity (mg g−1), C 0 and C e are the initial and final concentrations of the Th(IV) (mg L−1), V is the adsorbed solution volume (L), and m is the mass of the EB β-CD (g). All experiments were performed in three equal pieces.
Results and discussion
Characterization of 6-EA-β-CD and EB β-CD
Figure 3a, b, c shows the IR spectra of β-CD (1), 6-EA-β-CD (2) and EB β-CD (4). It can be seen that the characteristic absorption peak of OH group of β-CD is located at 3356 cm−1. The absorption peak intensity increases and the absorption peak position shifts from 3356 to 3422 cm−1 in the case of 6-EA-β-CD (2). This can be attributed to the presence of absorption band of NH2 stretching at 3400 cm−1 that overlaps with the absorption of OH group (in the range of 3200–3500 cm−1). And 6-EA-β-CD (2) appeared characteristic bands at 3422 cm−1 (σN–H), 1640 cm−1 (δN–H, NH2), 1108 cm−1 (σC–N); the characteristic peak of EB β-CD (4) have obviously enhanced at 3365 cm−1 (σN–H), 1659 cm−1 (δN–H, NH), 1109 cm−1 (σC–N), and there is red shift phenomena.
Figure 4a, b shown the 1HNMR (DMSO-d6, 400 MHz, TMS) spectrum of the 6-EA-β-CD (2) and EB β-CD (4). The characteristic peak of (a) δ: 5.71 (s, 9H), 4.83 (d, J = 3.3 Hz, 4H), 4.46 (s, 2H), 3.65 (d, J = 8.9 Hz, 10H), 3.57 (d, J = 10.2 Hz, 5H), 3.42–3.25 (m, 41H), 2.89 (s, 1H), 2.73 (s, 1H), 2.50 (solvent peak); (b) δ: 5.67(s, 15H), 4.82(d, J = 3.2 Hz, 19H), 4.43 (s, 8H), 3.68–3.63 (m, 28H), 3.58 (dd, J = 15.5, 10.1 Hz, 48H), 3.29 (d, J = 3.2 Hz, 21H), 2.89 (s, 1H), 2.73 (s, 1H), 2.50 (solvent peak). 1HNMR spectrum preliminary evidence to support successful synthesis of 6-EA-β-CD (2) and EB β-CD (4).
Elemental analysis. EB β-CD (4): the theoretical value calcd for C86H143O69N: C, 45.00 %; H, 6.24 %; N, 0.61 %. Actually found: C, 44.92 %; H, 6.90 %; N, 0.58 %. The detection result is basically consistent with the theoretical calculation of EB β-CD (4). The above test results adequately proved the successful synthesis of EB β-CD (4).
The surface area of the synthetic EB β-CD (4) and its porosity were determined from the adsorption isotherms of the material at the temperature of liquid nitrogen (77.3 K) using a Quantachrome NOVA 2200e instruments. The surface area was calculated using the Brunauer–Emmett–Teller (BET) method while pore size distribution using the Barett–Joyner–Halenda (BJH) model.
It can be seen from Fig. 5, the adsorption–desorption isotherms of the two substances present hysteresis loop, are IV type adsorption–desorption isotherms. At low relative pressure, the amount of nitrogen adsorbed increases with the increase of the pressure, which is monolayer adsorption. Relative pressure is about 0.2, the adsorption amount of N2 monomolecular layer being saturated, meanwhile the multi-molecular layer adsorption occurring, and the interaction force between adsorbate and sorbent is stronger than that’s of adsorbate. With the relative pressure increasing, the adsorption layers and the adsorption amount have increased. As the relative pressure of the isotherms of EB β-CD and β-CD is 0.4, 0.05, respectively, the capillary condensation phenomenon appeared. The larger the pore size is, the higher relative pressure that the capillary condensation phenomenon occurred is, which is coincide with the measured data (Table 1) by BJH model.
From Fig. 6, the larger the slope of the curve is, the more active the pore size distribution is. Apparently, the pore size of EB β-CD is larger than β-CD’s, and the range of the pore size distribution of the EB β-CD mainly in 2–10 nm, which proved the synthetic EB β-CD (4) is the typical mesoporous material [20]. So the EB β-CD will have great practical significance as the adsorbent material.
SEM spectra of EB β-CD (b) (4) and β-CD (a) (1) is shown in Fig. 7. The surface of EB β-CD is very crude and rugged while that of β-CD is much smooth and the bridged β-cyclodextrins (EB β-CD) have stronger adhesion. This is mainly due to the surface area of the bridged β-cyclodextrins (EB β-CD) is much larger than that of β-cyclodextrin. Which fit with what the measured data (Table 1).
Effect of pH
The pH value of the solution is an important factor in the study of thorium(IV) ion adsorption because it affects the species of metal ion that are present in solution. To assess the effect of pH solution on the adsorption process of Th4+ ion the experiments were carried out on a range of pH between 2 and 7 at room temperature, the initial concentration of Th4+ is 25 mg L−1, the amount of EB β-CD is 20 mg, the contact time is 60 min. From Fig. 8 we can see that the adsorbance of EB β-CD on thorium has changed vary with the acidity. At low pH value, it’s easier to hydrogen ion generate chelation with secondary amine [21]. Variation of adsorption with the increase of pH values could be explained by the presence of various compounds of thorium hydrolysis of the form Th(OH)3+, Th(OH) 2+2 , Th2(OH) 6+2 occurring at different pH values [22], which influence the adsorption effect result in adsorption illusion or error [23]. At pH 4, this was not sharply increased for apparent adsorbance of thorium, the reason for the phenomenon may be the influence of ionic strength and the stability of the formed complexes [24]. The mechanism of formation of the complexes is shown in the Fig. 9.
Effect of sorbent mass
A major factor affecting the efficiency of adsorption from an economic point of view is the mass of used sorbent (EB β-CD). The effect of different sorbent mass on adsorption process was studied at a fixed concentration (25 mg L−1) of thorium, pH value 4.0 and at room temperature, the contact time is 60 min. Figure 10 shows that with increasing sorbent mass from 10 to 30 mg the adsorbance of metal ion increases. The explanation would be that a greater mass of sorbent involves a larger number of functional groups and the synergy of EB β-CD double cavity.
Effect of contact time and kinetic studies
The influence of contact time on thorium ions adsorption was studied in a range from 15 to 120 min. These experiments were conducted at a pH of 4.0 for thorium ions, at a temperature of 298 K and a radioactive Th4+ concentration of 25 mg L−1. It can be observed from Fig. 11 that the sorption increases with increasing contact time and equilibrium is reached after a period of 60 min after which the sorption nearly remains constant. The largest adsorbance of Th4+ is 10.49 mg g−1. Compared with the study of Liu Peng [2], the time of the adsorption equilibrium was greatly shortened.
Pseudo-second-order models were employed to describe the adsorption process. The adsorption data were treated according to the pseudo-second-order kinetics using the following equation [25]:
where K s is the overall rate constant of the pseudo-second-order equation. Q e and Q t are the corresponding adsorption quantity of Th4+ when the adsorption equilibrium and t time, respectively. The plot of t/Q t versus t gives a straight line with slope and intercept equal to 1/Q e and 1/K s Q 2e , respectively. The fitness of the straight lines in Fig. 12 is better, the calculated Q e and K s are 11.558 mg/g and 0.00692 g mg−1 min−1, respectively, according to the equation of slope and intercept. The linear correlation coefficient (R 2 = 0.99415) of the pseudo-second-order equation is close to 1, which showed that the adsorption of bridged cyclodextrin (EB β-CD) on thorium is very good with the pseudo-second-order kinetics model. According to pseudo-second-order kinetics, the adsorption of Th(IV) indicating the influence of textural properties of EB β-CD on the rate of adsorption.
Effect of Th4+ concentration and adsorption isotherm
The effect of Th4+ initial concentration on the adsorption was also studied. Studies were performed at room temperature, ion concentration ranged from 5 to 30 mg L−1 while all the other arguments were maintained constant (pH 4, contact time 60 min, sorbent mass 20 mg, volume of radioactive solution 10 mL). From Fig. 13 it can be noted that the adsorption increases with increasing radioactive thorium ion concentration up to a value of 25 mg L−1 and then remains relatively constant. This variation can be explained by the fact that after a certain amount of sorbent, binding centers become saturated and no additional ions can be adsorbed.
The equilibrium data were modeled using the two frequently used isotherm models, namely Langmuir and Freundlich isotherms, to explain the experimental results and the real sorption behavior. The Langmuir model assumes that the adsorption of metal ions occurs on an energetically homogenous surface by mono-layer sorption and there are no interactions between the adsorbates on adjacent sites. The linear equation of the Langmuir adsorption model is expressed by the following equation [26]:
where C e is the equilibrium concentration of thorium ions in solution (mg/L), Q e is the amount adsorbed at C e (mg/g), Q m is the maximum adsorption capacity (mg/g), and K a is the binding constant which is related to the energy of adsorption (L/mg). A plot of 1/Q e against 1/C e was drawn. 1/Q m was calculated from the intercept and 1/Q m K a was calculated from the slope. The values of K a and Q m were obtained from Fig. 14, are 0.46 L/mg and 14.3 mg/g, respectively.
The Freundlich isotherm model is an empirical relationship describing the adsorption and assumes that different sites with several adsorption energies are involved (the surface of adsorbent is heterogeneous), and the linear equation is given by the following equation [25]:
where Q e (mg/g) and C e (mg/L) are the equilibrium concentrations of metal ion in the solid and liquid phase, respectively, and K F (mg/g) and n are characteristic constants related to the relative adsorption capacity of the sorbent and the intensity of adsorption, respectively. The plot of lnQ e against lnC e was drawn and found to be linear, which 1/n was calculated and the intercept equal to lnK F, respectively. Figure 15 shows the adsorption isotherms for Th(IV) ions, The Freundlich plot gave a slope less than 1, indicating nonlinear adsorption behavior with the concentration of Th(IV) in the concentration range studied. The observed values of K F and n of Th(IV) were found to be 4.4 (mg/g) and 2.2, respectively.
The comparison between the linear correlation coefficient (R) of Figs. 13 and 14 can be found that the adsorption process of EB β-CD on thorium is more consistent with the Langmuir isotherm model.
Study on adsorption thermodynamics
The temperature is one of the most important parameters on adsorption system, which can influence the adsorption behavior of thorium. The effect of temperature on the adsorption of EB β-CD on Th(IV) were investigated at 293, 308, 323, and 338 K, respectively. The other arguments were maintained constant (pH 4, contact time 60 min, sorbent mass 20 mg, volume of radioactive solution 10 mL). According to the following formula (5) [27], the static adsorption of the experiment was studied at different temperatures.
C 0 (mg/L) is the initial concentration of thorium ions; C e (mg/L) is the equilibrium concentration of thorium ions; m (g) is the quantity of EB β-CD; V (mL) is the volume of solution of thorium ions; K d (mL/g) is the partition coefficient; T (K) is absolute temperature; R is gas constant (8.314 J mol−1 K−1).
Thermodynamic parameters (ΔS 0 and ΔH 0) were calculated from the intercept and slope of the linear variation of lnK d versus 1/T (Fig. 16). All the results are summarized in Table 2. The Gibbs free energy of adsorption reaction (ΔG0) was calculated using the following relation [26]:
From Fig. 15 can be seen, distribution coefficient decrease with the rise of the temperature. But the slope of the curve is not great, and the temperature has little effect on the distribution coefficient. The adsorption behavior of the EB β-CD on Th(IV) can be carried out at room temperature. Thermodynamic parameters were evaluated to assess the thermodynamic feasibility and to confirm the nature of the adsorption process. ΔH 0 < 0, ΔG 0 < 0 (Table 2), so the positive values of ΔH 0 and ΔG 0 conform that the adsorption process is exothermic, spontaneous process.
Conclusions
In this work, we synthesized a specific bridged β-cyclodextrins (EB β-CD) using functional 6-OTs-β-CD as the nucleophilic reagent and 6-EA-β-CD as the carbyne. The EB β-CD (4) was confirmed by FTIR, 1HNMR, elemental analysis and SEM. It was found that specific surface area of EB β-CD (4) and raw β-cyclodextrin (1) is 8.190, 4.130 m2 g−1, respectively, from the adsorption–desorption isotherms and the corresponding pore diameter is 4.869, 3.389 nm, separately, from the pore size distribution curves by BJH model. The EB β-CD is the typical mesoporous material, which was successfully used for the adsorption of Th(IV). The adsorbent (EB β-CD) in this study had good adsorption capacity of Th(IV) at pH 4, and the adsorption equilibrium could reach a balance in 60 min. The adsorption quantity of Th(IV) was 10.49 mg g−1. Therefore, the bridged β-cyclodextrins will have a great application in the reclamation of radioactive metals (Th4+, UO2+ et al.) in waste water as an adsorbent material. The adsorption of Th(IV) was found to proceed according to pseudo-second-order kinetics indicating the influence of textural properties of EB β-CD on the rate of adsorption. The thermodynamic parameters obtained showed that the adsorption process is exothermic, spontaneous process.
References
Lin P, Guo L, Chen M (2014) Adsorption and fractionation of thorium and protactinium on nanoparticles in seawater. Mar Chem 162:50–59
Liu P, Qi W, Du Y, Li Z, Wang J, Bi J, Wu W (2014) Adsorption of thorium(IV) on magnetic multi-walled carbon nanotubes. Sci China Chem 57(11):1483–1490
Piech R, Paczosa-Bator B (2014) New adsorptive stripping determination of ATP with thorium(IV) on renewable silver amalgam film electrode. Int J Electrochem Sci 9:4287–4296
Aly A, Amer HA, Shawky S, Kandil AT (2014) Separation of thorium from aqueous solution by non living water hyacinth roots. Tech J Eng Appl Sci 4:1–13
Humelnicu D, Blegescu C, Ganju D (2014) Removal of uranium(VI) and thorium(IV) ions from aqueous solutions by functionalized silica: kinetic and thermodynamic studies. J Radioanal Nucl Chem 299:1183–1190
Sadeek SA, Moussa EMM, El-Sayed MA (2014) Uranium(VI) and thorium(IV) adsorption studies on chelating resin containing pentaethylenehexamine as a functional group. J Dispers Sci Technol 35(7):926–933
Zhou L, Jia Y, Peng J, Liu Z, Al-Zaini E (2014) Competitive adsorption of uranium(VI) and thorium(IV) ions from aqueous solution using triphosphate-crosslinked magnetic chitosan resins. J Radioanal Nucl Chem 302(1):331–340
Ji XZ, Liu HJ, Wang LL, Sun YK, Wu YW (2012) Study on adsorption of Th(IV) using surface modified dibenzoylmethane molecular imprinted polymer. J Radioanal Nucl Chem 295(1):265–270
Liu Y, Chen Y (2006) Cooperative binding and multiple recognition by bridged bis(β-cyclodextrin)s with functional linkers. Acc Chem Res 39(10):68–691
Zhao Y, Liu X-Q, Gu J (2008) Synthesis of novel bis(β-cyclodextrin)s linked with aromatic diamine and their molecular recognition with model substrates. J Phys Org Chem 21(6):440–448
Zhang Y-H, Zhang Y-M, Chen Y, Yang Y, Liu Y (2014) Phenanthroline bridged bis(β-cyclodextrin)s/adamantane-carboxylic acid supramolecular complex as an efficient fluorescence sensor to Zn2+. Org Chem Front 1(4):355–360
Kremer C, Lutzen A (2014) Allosteric binding of capsaicin by a bis(beta-cyclodextrin)-2,2′-bipyridine receptor. Chem Eur J 20(29):8852–8855
Zhang YM, Wang Z, Chen Y, Chen HZ, Ding F, Liu Y (2014) Molecular binding behavior of a bispyridinium-containing bis(beta-cyclodextrin) and its corresponding [2]rotaxane towards bile salts. Org Biomol Chem 12(16):2559–2567
Liu Y, You C-C, Li B (2001) Synthesis and molecular recognition of novel oligo(ethylenediamino) bridged bis(β-cyclodextrin) s and their Copper(ii) Complexes: enhanced molecular binding ability and selectivity by multiple recognition. Chem Eur J 7(6):1281–1288
Gangadhar T, Bhoi VI, Kumar S, Murthy CN (2013) Supramolecular self-assembly and nanoencapsulation of [60]fullerene by bis-β-cyclodextrin. J Incl Phenom Macrocycl Chem 79:215–223
Casas-Solvas JM, Ortiz-Salmeron E, Fernandez I, Garcia-Fuentes L, Santoyo-Gonzalez F, Vargas-Berenguel A (2009) Ferrocene-beta-cyclodextrin conjugates: synthesis, supramolecular behavior, and use as electrochemical sensors. Chem Eur J 15(33):8146–8162
Yin JJ, Sharma S, Shumyak SP, Wang ZX (2013) Synthesis and biological evaluation of novel folic acid receptor-targeted, beta-cyclodextrin-based drug complexes for cancer treatment. PLoS One 8(5):1–20
Sun M, Zhang H-Y, Liu B-W, Liu Y (2013) Construction of a supramolecular polymer by bridged bis(permethyl-β-cyclodextrin) s with porphyrins and its highly efficient magnetic resonance imaging. Macromolecules 46(11):4268–4275
Liu Y, You C-C, Inoue Y (2000) Molecular recognition of fluorescent dyes with novel triethylenetetraamine-tethered bis(β-cyclodextrin) and its copper(II) complex: enhanced binding and selectivity by tether ligation. Tetrahedron Lett 41:6869–6873
Zhao Z-G (2004) Surface fractal analysis of mesoporous adsorbents. Acta Chim Sin 62:219–223
Zhao D (2008) Adsorption of thorium(IV) on MX-80 bentonite: effect of pH, ionic strength and temperature. Appl Clay Sci 41:17–23
Yang SK, Tan N, Yan XM, Chen F, Long W, Lin YC (2013) Thorium(IV) removal from aqueous medium by citric acid treated mangrove endophytic fungus Fusarium sp. #ZZF51. Mar Pollut Bull 74(1):213–219
Tong KS, Kassim MJ, Azraa A (2011) Adsorption of copper ion from its aqueous solution by a novel biosorbent Uncaria gambir: equilibrium, kinetics, and thermodynamic studies. Chem Eng J 170(1):145–153
Sheng G, Hu J, Wang X (2008) Sorption properties of Th(IV) on the raw diatomite–effects of contact time, pH, ionic strength and temperature. Appl Radiat Isot 66(10):1313–1320
Zafar S, Khalid N, Daud M, Mirza ML (2015) Kinetic studies of the adsorption of thorium ions onto rice husk from aqueous media: linear and nonlinear approach. Nucleus 52(1):14–19
Mellah A, Chegrouche S, Barkat M (2006) The removal of uranium(VI) from aqueous solutions onto activated carbon: kinetic and thermodynamic investigations. J Colloid Interface Sci 296(2):434–441
Zhao D (2008) Adsorption of thorium(IV) on MX-80 bentonite: effect of pH, ionic strength and temperature. Appl Clay Sci 41(1–2):17–23
Acknowledgments
The authors gratefully acknowledge the financial support from National Natural Science Foundation of China (No. 11375084).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, C., Liu, Hj., Peng, L. et al. Synthesis of ethylamine-bridged β-cyclodextrins and adsorption properties of thorium. J Radioanal Nucl Chem 308, 251–259 (2016). https://doi.org/10.1007/s10967-015-4306-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4306-z