Abstract
Adsorption behavior of uranyl and thorium ions from synthetic radioactive solutions onto functionalized silica as sorbent has been investigated. The effect of contact time, initial concentration of radioactive solutions, sorbent mass, pH value and temperature on the adsorption capacity of the sorbent was investigated. Negative values of Gibbs free energy of adsorption suggested the spontaneity of the adsorption process on both functionalized silica with –NH2 groups and with –SH groups. Positive values obtained for ΔH° indicates that the adsorption is an endothermic process. The adsorption isotherms were better fitted by Freundlich model and the adsorption kinetic was well described by the pseudo-second order equation. Desorption studies indicated that the most favorable desorptive reagents for UO2 2+ is HNO3 1 M and for Th4+ is EDTA 1 M solutions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Removal and recovery of radioactive elements used in nuclear fuel is becoming an important aspect in terms of environmental protection. Removal of radioactive ions from wastewater is a major problem in the treatment of liquid waste because these ions are harmful to the environment and human health due to their high toxicity, even at low concentrations, and their long half lives. These pollutants appear in water from some industrial activities such as mining, nuclear power plants, nuclear weapons, laboratory activities. Several methods have been developed to remove radioisotopes from waste water including: ion exchange, membrane-related process, biological process and electrochemical technique [1–5].
Adsorption represents an effective and convenient technique used in the separation and recovery of various heavy and radioactive metals. This method has the advantage of separating small quantities of substance from large volumes of solution.
Over the last decade, the use of inorganic materials as sorbents for removal of radioactive and heavy metals in sorption processes has been used extensively [6–9].
Recently, Shi et al. [10] reported the use of nanomaterials and nanotechnologies for applications in nuclear environmental remediation. These materials have a number of advantages, such as: a large specific surface, good chemical stability, resistance to radiations, many possibilities to functionalization. In earlier studies Liu et al. [11] reported the use of silica nanoparticles (Santa Barbara Amorphous type material, or SBA-15) amino functionalized (APSS) as a rapid and efficient material for the removal of U(VI) from the environment.
Inorganic materials have proven to be advantageous due to their low cost and their high efficiency. An important requirement for an advantageous sorption is for the sorbent to be sufficiently selective with high sorption capacity, resistant to high temperatures and radiation. Adsorption method is considered favorable for liquid waste remediation due to its simplicity and efficiency.
Most methods for removing radionuclides from contaminated waters are based on formation of complexes between radiocation species and sorbent [12].
The objective of this study was to examine the adsorption of uranyl and thorium ions from aqueous solutions on silica functionalized with –NH2 and –SH groups, in static system, based on various experimental parameters (contact time, pH, temperature, initial metal ion concentration and amount of sorbent).
Materials and methods
Chemicals
All substances and reagents used in these studies were of analytical purity. In all experiments distilled water was used for preparation and dilution of solutions.
Synthesis of sorbents
Silica functionalized with –SH groups
The synthesis was realized by a condensation between the –OH groups from the surface of the silica material and the methoxy groups of the (3-mercaptopropyl)-trimetoxysilane. In order to generate –OH acid groups on the surface of the silica an activation process is required. In order to do that, the silica material was kept in HCl 10 % solution and reflux for 12 h. After this activation step, the obtained material was washed with distilled water and methanol and dried for 12 h at 130 °C [13, 14].
For the condensation reaction, the activated silica was suspended in anhydrous toluene under nitrogen atmosphere and vigorous magnetic stirring. Over the suspension 5.4 mmol of (3-mercaptopropyl)-trimetoxysilane for each gram of silica material was added under magnetic stirring and then heated at reflux for 24 h. The obtained material was washed with methanol and methylene chloride, and Soxhlet extracted for 24 h with methylene chloride in order to deactivate all the acid –OH groups unreacted from the surface.
Silica functionalized with –NH2 groups
After the same activation process of the surface of the silica matrix, a condensation between aminopropyl-trietoxysilane and the active –OH groups was realized under nitrogen atmosphere and reflux temperature using as solvent anhydrous toluene. The obtained material was washed with methanol and Soxhlet extracted with methanol for 12 h.
The surface area of the silica matrix and its porosity were determined from the adsorption isotherms of the material at the temperature of liquid nitrogen (−196 °C) using a QUANTACHROME AUTOSORB NOVA 2200e apparatus. The surface area was calculated using the Brunauer–Emmett–Teller method while pore size distribution using the Barett-Joyner-Halenda model [15, 16].
Surface area determination
The BET surface area for both materials was calculated using the Brunauer–Emmett–Teller method. In both cases H4 type hysteresis loops were obtained that correspond to uniform “ink-bottle” shape pores. From the hysteresis loop (Fig. 1) it can be seen that the functionalization of the silica material pores occurred without collapsing the inner pores structure of the silica material.
The values for the specific values along with the pore volume of the obtained materials and the starting material are presented in the Table 1.
Sorption experiments
Adsorption experiments were carried out in static system for each ion by agitation the radioactive solution—sorbent mixture. Synthetic radioisotope solution was prepared by dissolving in distilled water, where a few drops of HNO3 had been added, the corresponding salts, UO2(NO3)2·6H2O (Merck) and Th(NO3)4·5H2O analytical grade. The experiments were performed on a pH range between 2 and 6, initial solution concentration ranged from 10 to 100 mg L−1, temperature of 298 K—318 K, and the mass of sorbent ranged from 0.01 to 0.04 g. The pH of the solution was adjusted with 0.1 M HNO3 and NaOH, respectively.
After establishing equilibrium sorption, the sorbent was separated by filtration and the concentration of radioisotope remaining in solution was determined spectrophotometrically by complexation with Arsenazo III [17, 18].
The percent sorption of radioactive ions from aqueous solution and the amount of metal adsorbed per unit mass of sorbent have been calculated in Ref. [19] and K d in Ref. [20]:
where C 0 is the initial concentration of metal (mg L−1), C e is the equilibrium metal concentration (mg L−1), V is the volume of solution (L) and m is the mass of sorbent (g).
Desorption experiments
Desorption processes are important from two points of view: first, the recovery of radionuclide and subsequent use of nuclear energy and, secondly the regeneration of sorbent for his reuse in other adsorption processes. Desorption was achieved in static system using sorbent loaded with radioactive metal after the sorption processes. For this purpose we used some desorption reagents such as: EDTA, HNO3, HCl and Na2CO3. Contact time between desorption reagents and sorbent with radioactive ions was 24 h in all experiments.
Metal ion concentration in the supernatant was determined by spectrophotometric method with Arsenazo III. The percentage of desorbed radioactive ion was calculated with the equation:
where amountdes represents the amount of desorbed radioactive ions and amountads is the amount retained on the sorbent.
All experiments were carried out in duplicate with a standard error of 5 %.
Results and discussion
Effect of contact time
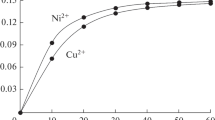
The influence of contact time on radioactive ions adsorption on the two sorbents was studied in a range from 0 to 60 min. These experiments were conducted at a pH of 4.0 for thorium and 5.0 for uranyl ions, at a temperature of 298 K and a radioactive ion concentration of 60 mg L−1.
It can be noted from Fig. 2 that the sorption increases with increasing contact time and equilibrium is reached after a period of 45 min after which the sorption remains constant. It is found that the adsorption process onto silica functionalized with amino groups is more intense in comparison with silica functionalized with –SH groups.
Effect of metal concentration
The effect of metal concentration on uranium and thorium retention was also studied. Studies were performed at room temperature, ion concentration ranged from 10 to 100 mg L−1 while all the other parameters were maintained constant (pH 4 and 5, respectively, contact time—40 min, sorbent mass—0.025 g, volume of radioactive solution—0.080 L). From Fig. 3 it can be observed that the sorption increases with increasing radioactive ion concentration up to a value of 60 mg L−1 and then remains relatively constant. This variation can be explained by the fact that after a certain concentration of sorbent, binding centers become saturated and no additional ions can be adsorbed [21].
Effect of sorbent mass
A major factor affecting the efficiency of adsorption from an economic point of view is the mass of used sorbent. The adsorption process is not effective if it requires a large amount of sorbent. The effect of different sorbent mass on adsorption process was studied at a fixed concentration of radionuclide of 60 mg L−1, pH value 5.0 (for uranium) and 4 (for thorium) and at a temperature of 298 K. Figure 4 shows that with increasing sorbent mass from 0.01 to 0.04 g the percentage of metal retained increases. The explanation would be that a greater mass of sorbent involves a larger number of active centers on the surface.
Effect of pH
The pH value of the solution is an important parameter in the study of radioactive ions adsorption because it affects the species of metal ions that are present in solution. To assess the effect of pH solution on the adsorption process of radioactive ions the experiments were carried out on a range of pH between 2 and 6. From Fig. 5 we can observe that the maximum adsorption capacity appear at 4 (for thorium ion) and 5 (for uranyl ion), respectively, that are in good agreement with similar studies [8, 9, 22–26].
Variation of adsorption with pH values could be explained by the presence of various compounds of uranium hydrolysis of the form [(UO2)p(OH)q](2p−q)+ occurring at different pH values [27]. At low pH value uranium exists in solution as ions UO2 2+(monomer), but with increasing pH uranyl ions undergo hydrolysis processes for obtaining species such as: [(UO2)2(OH)2]2+ (dimer), [(UO2)3(OH)5]+(trimer). Predominant species in acidic environments is [(UO2)2(OH)2]2+, and at pH values above five uranium may exist in the predominant form [(UO2)3(OH)5]+ [28].
Formation of hydroxocomplexes could be explained by the following equilibrium [26]:
Adsorption could be explained as a substitution reaction of some species (HO−, H2O) of the radionuclide coordination sphere with –NH2 or –SH group from the support [29–31].
Liu [11] mentioned that at lower pH, the amino groups are protonated and the radioactive ions are not binding to sorbent due to the electrostatic repulsion, leading to lower sorption capacities [8]. As pH increases, the amino groups are deprotonated gradually and appears electrostatic interaction between N from amino group and U(VI) ions that leads to an increase of sorption capacity.
A pH value beyond six leads to polymerization of the hydrolyzed uranyl ions [27, 32, 33].
Thermodynamic studies
The effect of temperature on adsorption processes of radioactive ions for the two types of sorbents was investigated at three temperatures (298, 308, 318 K) and the results are shown in Fig. 6. The increase of the percentage of metal adsorbed with increasing temperature indicates the endothermic nature of this process. This variation can be explained by the fact that increasing temperature increases the rate of diffusion of sorbate molecules along the outer layer and the internal pores of sorbent particles [34, 35].
Thermodynamic parameters: standard free energy (ΔG°), enthalpy (ΔH°) and entropy of the system (ΔS°) were estimated using Eqs. (5) and (6):
where K d is distribution coefficient estimated from Eq. (3).
The obtained data from the study of the influence of temperature on adsorption were used in order to determine these thermodynamic parameters. The values of ΔH° and ΔS° were calculated from the slope and intercept with the plot lnK d versus 1/T (Fig. 7). The results are presented in Table 2.
Positive values obtained for ΔH° indicate that the adsorption is endothermic process, while negative values of Gibbs energy indicate the spontaneity of adsorption process.
Adsorption isotherms
Adsorption isotherms are important in understanding the mechanism of adsorption and the interaction between sorbent and sorbate. To study the adsorption of U and Th onto the sorbent three types of isotherm models were used: Langmuir, Freundlich and Dubinin–Radushkevich [36]. Langmuir adsorption characterizes an adsorption monolayer on a surface with a finite number of identical centers that are homogeneously distributed on the surface of sorbent. In these studies we applied linearised form of Langmuir isotherm:
where q e is the amount of metal adsorbed per unit of sorbent (mg g−1), C e is the equilibrium concentration of metal ion (mg L−1), q m is a parameter that gives the maximum adsorption capacity of the sorbent (mg g−1), K L is a constant that refers to the energy of adsorption/desorption (L mg−1). K L and q m values were calculated from intercept, or slope of the plot C e/q e versus C e (Fig. 8).
The characteristics of Langmuir isotherms can be expressed by a dimensionless constant so-called equilibrium parameter or separation factor [37]:
K L is the Langmuir constant and C 0 is the initial concentration of radioactive ions.
For a favorable adsorption the R L value must be between 0 and 1. In this respect, if R L > 1 adsorption is unfavorable, and if R L = 0 adsorption is irreversible. In the present studies, the obtained R L values were less than one (Table 3) which shows that the adsorption processes were favorable.
The Freundlich isotherm is the second mathematical model used to describe the adsorption metal present in solution on solid surface. This model describes a heterogeneou s surface adsorption. The linearised form of this model is given by the Eq. (9):
where q e is the amount of radiocation adsorbed at equilibrium (mg g−1); C e concentration of metal ion in solution at equilibrium (mg L−1), K F (L mg−1) and 1/n are the Freundlich constants.
The values of 1/n and K f can be calculated from the slope and intercept of the linear plot of log q e versus log C e (Fig. 9), and are given in Table 3. The values of 1/n were less than one indicating a favorable adsorption.
The Dubinin–Radushkevich isotherm was applied to determine apparent free energy of adsorption [38].
The linearised form of the isotherm can be written as:
where K DR is a constant that relates to the adsorption energy (mol2 J−2), X m is a constant indicating the adsorption capacity of the sorbent (mg g−1).
The adsorption potential is independent from temperature but depends on the nature of sorbent and sorbate. Polanyi’s potential, ε, can be calculated with the expression:
Free energy of adsorption (E) can be calculated using the following equation:
The slope of the plot of ln q e versus ε2 gives K DR (mol2 J−2) and the intercept yields the adsorption capacity, q m (mg g−1). The results are given in Table 3.
Table 4 points out a comparison of the uranyl and thorium ions adsorption capacity of some sorbents. It will be seen that the adsorption capacity of functionalized silica with –NH2 and –SH groups was lower that of cross linked chitosan (CS) beads and chitosan/clinoptilolite (CS/CPL) composite but was several times higher that of magnesium silicate hollow spheres. Hence, functionalized silica with –NH2 and –SH groups could be a promising adsorbent for the removal U(VI) and Th(IV) ions from waste waters.
Kinetic studies
The adsorption kinetic of uranyl and thorium ions was investigated by applying pseudo-first order and pseudo-second order models.
The linearised forms of the two models are expressed by the equations:
and
The kinetic parameters for the adsorption process were evaluated from the slope and intercept of the respective linear plots and are presented in Table 5.
The results obtained from the analysis of experimental data indicated that the adsorption of uranyl and thorium ions on modified silica is best described by pseudo-second order model.
Desorption studies
The sorbent loaded with radioactive ion has been treated with a series of desorption reagents for the recovery of radioactive ion. Contact time between the desorption reagent and adsorbent material loaded with radioactive ion was of 24 h. In Table 6 are presented the results of desorption processes.
Analyzing the results obtained, it can be observed that for the recovery of uranyl ions a 1 M HNO3 solution can be used and for the recovery of thorium ions from the same sorbent it is appropriate to use a solution of 1 M EDTA.
Conclusions
It was studied the adsorption of uranyl and thorium ions on sorbents of silica type functionalized with groups such as –SH and –NH2.
Temperature variation was used to calculate the thermodynamic parameters that characterize the adsorption process. The data obtained for these parameters (ΔH° and ΔG°) suggest that the adsorption process is endothermic and spontaneous.
The correlation coefficients indicate that the Freundlich model conformed better than Langmuir and Dubinin–Radushkevich models for the adsorption of the two ions.
In view of high sorption capacity of these materials they could be used in the construction of artificial barriers that prevent penetration of uranium in underground waters.
As a result of desorption studies it was found that the recovery of uranyl ions from sorbent can be successfully done using a solution of HNO3 and a solution of 1 M EDTA for thorium ions.
References
Mahramanlioglu M (2003) J Radioanal Nucl Chem 256:99–105
Tsuruta T (2002) J Biosci Bioeng 94:23–28
Someda HH, Sheha RR (2008) Radiochemistry 50:56–63
Ladeira AC, Morais CA (2005) Miner Eng 18:1337–1340
Cecal A, Gulea A, Rudic V, Palamaru I, Humelnicu D, Popa K (1997) Isot Environ Health Stud 33:327–331
Akyil S, Aslani MAA, Eral M (2003) J Radioanal Nucl Chem 256:45–51
Aytas S, Akyil S, Eral M (2004) J Radioanal Nucl Chem 260:119–125
Khazaei Y, Faghihian H, Kamali M (2011) J Radioanal Nucl Chem 289:529–536
Akkaya R, Akkaya B (2013) J Nucl Mater 434:328–333
Shi W-Q, Yuan L-Y, Li Z-J, Lan J-H, Zhao Y-L, Chai Z-F (2012) Radiochim Acta 100(8–9):727–736
Liu YL, Yuan LY, Yuan YL, Lan JH, Li ZJ, Feng YX, Zhao YL, Chai ZF, Shi WQ (2012) J Radioanal Nucl Chem 292:803–810
Donia AM, Atia AA, Moussa EM, El-Sherif AM (2009) Abd El-Magied MO. Hydrometallurgy 95:183–189
Blegescu C, Ganju D, Palamaru MN (2012) Rev Roum Chim 57(7–8):769–773
Blegescu C, Ganju D, Palamaru MN (2012) Rev Roum Chim 57(4–5):415–419
Di Bernardo P, Zanonato PL, Tamburini S, Vigato PA (2007) Inorg Chim Acta 360:1083–1094
Saikia L, Srinivas D, Rarnasamy P (2007) Micropor Mesopor Mater 104:225–235
Kilincarslan A, Akyil S (2005) J Radioanal Nucl Chem 264:541–548
Khan MH, Ali A, Khan NN (2001) J Radioanal Nucl Chem 250:353–357
Gao L, Yang ZQ, Shi KL, Wang XF, Guo ZJ, Wu WS (2010) J Radioanal Nucl Chem 284:519–526
Kulik D (2002) Radiochim Acta 90:815–832
Talip Z, Eral M, Hiçsönmez Ü (2009) J Environ Radioact 100:139–143
Donat R (2009) J Chem Thermodyn 41:829–835
Nilchi A, Shariati Dehaghan T, Rasouli Garmarodi S (2013) Desalination 321:67–71
Yi Z, Yao J, Wang F, Chen H, Liu H, Yu C (2013) J Radioanal Nucl Chem 295:2029–2034
Kutahyalı C, Eral M (2010) J Nucl Mater 396:251–256
Zareh MM, Aldaher a, Hussein AEM, Mahfouz MG, Soliman M (2013) J Radioanal Nucl Chem 295:1153–1159
Baes JrC F, Mesmer RE (1976) The hydrolysis of cations. Wiley, New York, p 489
Fan F, Ding H, Bai J, Wu X, Lei F, Tian W, Wang Y, Qin Z (2011) J Radioanal Nucl Chem 289:367–375
Tan XL, Fan QH, Wang XK, Grambow B (2009) Environ Sci Technol 43:3115–3121
Chen CL, Xu D, Tan XL, Wang X (2007) J Radioanal Nucl Chem 273:227–233
Hu J, Chen CL, Sheng GD, Li XJ, Chen YX, Wang XK (2010) Radiochim Acta 98:421–429
Misaelides P, Godelitsas A, Filippidis A, Charistos D, Anousis C (1995) Sci Total Environ 173(174):237–246
Shuibo X, Chun Z, Xinghuo Z, Jing Y, Xiaojian Z, Jingsong W (2009) J Environ Radioact 100:162–166
Toth LM, Begun GM (1981) J Phys Chem 85:547–549
Mishra SP, Tiwari D (2002) J Radioanal Nucl Chem 251:47–53
Foo KY, Hameed BH (2010) Chem Eng J 156:2–10
Weber TW, Chakkravorti RK (1974) AIChE J 20:228–232
Dubinin MM, Radushkevich LV (1947) Chem Zent 1:875–889
Humelnicu D, Dinu MV, Dragan ES (2011) J Hazard Mater 185:447–455
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Humelnicu, D., Blegescu, C. & Ganju, D. Removal of uranium(VI) and thorium(IV) ions from aqueous solutions by functionalized silica: kinetic and thermodynamic studies. J Radioanal Nucl Chem 299, 1183–1190 (2014). https://doi.org/10.1007/s10967-013-2873-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-013-2873-4