Abstract
In present study it is aimed to radiolabel vincristine with 99mTc and to evaluate bioaffinity of 99mTc labeled vinc. The optimum conditions required to obtain 99.6 ± 0.4 %, (n = 5) radiolabeling yield of 99mTc-vincristine (99mTc-vinc) were as follows: pH 4, 5 µg of vincristine sulphate, 6 µg SnCl2·2H2O as a reducing agent and 10 min incubation time at room temperature. Quality control of 99mTc-vinc was done by using paper electrophoresis and thin layer chromatography. The radiolabeling yield was confirmed by High performance liquid chromatography using radioactive and UV detector operating at 230 nm. 99mTc-vinc was stable in vitro for 5 h. Biodistribution and scintigraphy of 99mTc-vinc was performed in mice and rabbits respectively and that 99mTc-vinc showed high uptake of it in liver and spleen. Finally 99mTc-vinc may be the potential imaging agent for liver and spleen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nuclear medicine is unique medical modality that practices radiopharmaceuticals for imaging and therapy. The medical imaging uses molecular probes and radiotracers for diagnosis of tumor metabolism, proliferation and other specific targets thus enabling the early detection of diseases [1]. The imaging modalities for detection and characterization are ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI), Single Photon emission Tomography (SPECT) and positron emission tomography (PET) [2]. 99mTc as a routinely SPECT imaging radiotracer, various 99mTc-labeled compounds have been prepared for imaging purposes and some of them are routinely used in diagnostic nuclear medicine [3–14]. For tracing the abnormalities of liver and spleen, the goal of scintigraphy is to empower the concerned physician to image hepatic and/or splenic tissue and thus helpful for determining the size and shape of the liver and spleen as well as for detecting functional abnormalities of the reticulo endothelial cells of these organs [15, 16]. 99mTc has become the most major radioisotope for SPECT medical imaging due to its ideal properties of short suitable half-life (6 h), 140 keV γ-rays emissions best suitable for scintigraphy and low radiation burden [17]. Most of the 99mTc-radiopharmaceuticals employed for imaging show similar pharmacokinetic properties in animals and human. A variety of radiopharmaceuticals have been described for diagnostics such as 99mTc-UDCA [18], 99mTc-disofenin (DISIDA), 99mTc-EHIDA [19], 99mTc-mebrofenin [20–23], 99mTc-lidofenin [24], 131I-AFP-MoAb [25] and 99mTc- MIBI [26], 99mTc-duramycin, [12] and 99m Tc-labeled bombesin analog [27]. For liver-splenic imaging, the agents labeled with 99mTc are sulfur colloid [28] and red blood cells [29].
A large number of drugs selectively target actively proliferating cells such as intercalating agents, anti-metabolites, mitotic inhibitors and DNA-alkylating agents. Vincristine, a mitotic inhibitor used in cancer chemotherapy is formally known as leurocristine and is a vinca alkaloid from the Catharanthus roseus. It is found to treat a large variety of cancers, such as sarcomas, acute leukaemia, breast cancer, malignant lymphoma, acute erythraemia, Hodgkin’s disease, and testicular cancer. Vincristine is a cell cycle-dependent compound that is administered via intravenous infusion and is used to inhibit cell cycle progression at M-phase [30–32].
In this work, the labeling and characterization of 99mTc-labeled vincristine (99mTc-vinc) was carried out for liver and spleen imaging in animal models by performing in vitro and in vivo assays followed by scintigraphy.
Materials and methods
Vincristine sulphate was obtained from Lahore Pakistan. 99mTc was obtained from a locally produced fission based PAKGEN 99Mo/99mTc generator. All chemicals used were AR grade. The approval for animal’s experiments was taken from the Animal Ethics Committee of the Pakistan Institute of Nuclear Science and Technology (Document no. IPDs-H-SOP-04-003).
Synthesis of 99mTc-vinc
Known amount of stannous chloride dihydrate was dissolved in 0.1 mL of concentrated HCl and diluted with distilled water to get required amount of reducing agent. To the varying amount of ligand (Vincristine sulphate); certain amount of SnCl2·2H2O and 10 mCi of \( ^{{ 9 9 {\text{m}}}} {\text{TcO}}_{4}^{ - } \) was added. The pH of the solution was adjusted with the diluted NaOH solution. The mixture was then incubated for different time periods at room temperature (25 °C) for labeling purposes. At least five set of experiments were performed for each point.
Quality control
Electrophoresis of 99mTc-vinc
Electrophoresis of radiotracer 99mTc-vinc was studied by using Deluxe electrophoresis chamber (Gelman) system. The phosphate buffer of pH 6.8 was used in this experiment. Whatman No. 1 paper of 30 cm was used marked with L at left side of the strip and R at right side of the strip. The strip was placed in the electrophoresis chamber containing buffer in such a way that left side dip at anode and right side at cathode; one drop of 99mTc-vinc was poured at the middle of the strip and electrophoresis was run for 45–60 min at a voltage of 300 V. After completion of electrophoresis, the strip was scanned by using 2π scanner to know the charge on 99mTc-vinc.
Thin layer chromatography of 99mTc-vinc
Radiochemical yield of 99mTc-vinc was checked by chromatographic method using Whatman No. 3 paper and ITLC-SG strips (Gelman Science). Free \( ^{{ 9 9 {\text{m}}}} {\text{TcO}}_{4}^{ - } \) in the preparation was determined by using Whatman No. 3 paper as the stationary phase and acetone as mobile phase. Reduced and hydrolyzed activity was determined by using instant thin layer paper chromatography (ITLC-SG strips) as the stationary phase and 0.5 M NaOH as a mobile phase. The distribution of labeled, free and hydrolyzed compounds on chromatographic strips was measured by a 2π Scanner (Berthold, Germany). Alternatively, the strips were cut into 1 cm segments and counted by a gamma-counter.
High performance liquid chromatography of 99mTc-vinc
HPLC of 99mTc-vinc was studied by using D-200 Elite HPLC system. Sodium Iodide crystal detector was used for radioactivity measurement. The column of C-18 (waters, µ-Bondapak™C18, 3.9 × 300 mm) was used as stationary phase and a mixture of acetonitrile and water were used as mobile phase in the ratio 80:20 (v/v %). The flow rate of the mobile phase was adjusted up to 1 mL per minute. UV detector was used for detection purpose and work was done at wavelength of 230 nm, while gamma detector (NaI) was used for monitoring of 99mTc activity.
In vitro stability study of 99mTc-vinc
After choosing suitable vincristine sulphate/99mTc ratio and pH, the complex was incubated for 24 h at room temperature. To observe the stability of the complex 99mTc-vinc after 30 min, 1, 2, 4, 6 and 24 h, the complex was spotted on paper strips, developed and scanned likewise by virtue of which in vitro stability of the labeled preparation was ascertained.
In vitro stability in normal human serum
1.8 mL of human serum was mixed with 0.2 mL (2 mCi) of 99mTc-vinc and incubated at 35 °C. 0.2 mL aliquots were withdrawn during the incubation at 1, 4 and 24 h and subjected to chromatography for determination of 99mTc-vinc, reduced/hydrolyzed 99mTc and free \( ^{{ 9 9 {\text{m}}}} {\text{TcO}}_{4}^{ - } \).
Biological distribution of 99mTc-vinc in mice
The biological distribution study was done using nine Swiss Albino mice divided into three groups (three animals in each group) weighing 30–35 g. Each anesthetized animal was injected in tail vein with 0.4 mL containing ~74 MBq (2 mCi) of 99mTc-vinc. The mice were sacrificed after anesthesia and biodistribution was determined. The sample of blood (1 mL) was taken by cardiac puncture, weighted and activity in total blood was calculated by assuming blood volume = 6.5 % of body weight. The whole animals were then weighed and dissected after 1, 4 and 24 h. Samples of muscle, liver, spleen, lungs, kidney, stomach, femur, heart and brain were removed, weighed and content of radioactivity was measured using a gamma counter. Corrections were made for background radiation and physical decay while performing the experiment. The results were expressed as the % uptake of injected dose per gram organ tissue.
SPECT-imaging
The imaging study was performed using Swiss Albino mice and healthy rabbits with weight range of 30–35 and 2.0–2.5 kg respectively. 99mTc-vinc (2 mCi) was injected intravenously into the tail of mice while 99mTc-vinc (3 mCi) was administered in the marginal ear vein of rabbit. The animal was sedated by one mL of intramuscular diazepam injection and was immobilized on gantry table under the head of gamma camera projecting the dorsal view of the animal. The energy window of 20 % was set on 140 keV. Images were acquired on 256 × 256 matrix size for 5 min each. Whole body static images were taken at 1, 4 and 6 h in rabbit while 1, 4 and 24 h in mice after 99mTc-vinc injection. Single headed Siemens Integrated Orbiter Gamma Camera System interfaced with high-resolution parallel-hole collimator was used to acquire digital images. (Macintosh® Operating System 7.5 Software used on the ICON™ Workstation).
Results and discussion
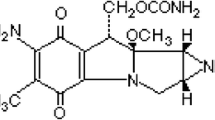
The Vincristine contains the sulphate salt of vinca alkaloid from the C. roseus formerly Vincarosea. It has molecular formula C46H56N4O10 as shown in (Fig. 1).
Synthesis of 99mTc-vinc
The synthesis of 99mTc-vinc was performed to determine the best conditions ensuring high radiochemical yield, high purity, and stability of 99mTc-vinc. The examination of the effect of the different parameters on the labeling yield such as amount of vincristine, pH of the reaction mixture, amount of SnCl2·2H2O, and reaction time was studied. The effects of pH are shown in Fig. 2. At low pH (2–3) the labeling efficiency was 60–80 %, while at pH 4 the labeling efficiency of 99mTc-vinc was ~100 %. In basic media 7–8 the labeling efficiency was decreased (~50 %). Hence further experiments were performed at pH 4.
The amount of reducing agent, SnCl2·2H2O, which gave the highest labeling efficiency, was 4–8 and 6 µg of SnCl2·2H2O was chosen (Fig. 3) to avoid colloid formation. Basically preparation of the variety of 99mTc radiopharmaceuticals involves reduction of 99mTc from 7+ to lower-valence state, which enables its chelation by compounds of diagnostic purposes. The complexation of 99mTc with 5 µg vincristine at pH 4 with 6 µg of SnCl2·2H2O gave a maximum labeling efficiency of 99.6 ± 0.4 within few minutes (Fig. 4). Labeling yield at different time intervals 5, 30 min, 1, 2, 4 and 6 h after the initiation of reaction came out to be 97.458 at initial 5 min and 100 % was maintained till 5 h. Graphical representation of these results is depicted in Fig. 5. The final formulation for the radiotracer 99mTc-vinc was: Vincristine sulphate 5 µg; SnCl2·2H2O 6 µg; pH ~4; 99mTc 370 MBq (10 mCi) and reaction mixture volume 1.5 mL. With the development of molecular biology based medicine, a transition is being carried out to incorporate into diagnostic interpretation information because of biochemical perturbations existing in the disease. Biomedical imaging technique can help to diagnose tumors, optimize drug development, and suggest response to a therapeutic modality [33].
Quality control and stability of 99mTc-vinc
The electrophoresis illustrate that complex is neutral in nature (Fig. 6). Labeling efficiency, radiochemical purity and stability were assessed by a combination of ascending chromatography and instant thin layer chromatography impregnated with silica gel. In paper chromatography using acetone as the solvent, free \( ^{{ 9 9 {\text{m}}}} {\text{TcO}}_{4}^{ - } \) moved towards the solvent front (Rf = 1), while 99mTc-vinc and reduced/hydrolyzed 99mTc remained at the point of spotting. In ITLC-SG chromatography using 0.5 M NaOH as solvent, reduced/hydrolyzed 99mTc remained at the point of spotting, whereas 99mTc-vinc and free \( ^{{ 9 9 {\text{m}}}} {\text{TcO}}_{4}^{ - } \) moved towards the solvent front. HPLC results of inactive ligand illustrate that ligand is >90 % pure (Fig. 7) HPLC analysis of 99mTc-vinc illustrate that ~100 % 99mTc binds with available ligand (Fig. 8). When the preparation (labeled radiopharmaceutical) was incubated with normal human serum at 35 °C, no significant increase in free \( ^{{ 9 9 {\text{m}}}} {\text{TcO}}_{4}^{ - } \) or reduced/hydrolyzed 99mTc was seen up to 24 h. The total impurities were found to be <5 % (Fig. 9).
Biodistribution and SPECT imaging in animals
Biodistribution of 99mTc-vinc in various organs of the mice at 1, 4 and 24 h after intravenous administration is presented in Table 1. The in vivo behavior of the 99mTc-vinc was expressed as percentage of injected dose per gram organ tissue (%ID/gram organ tissue). 99mTc-vinc is accumulated in spleen (~8 %), liver (>18 %), and kidney (>19 %) after 1 h post administration. Spleen showed significant uptake of 99mTc-vinc being the organ system with high cell turns over. The activity uptake of stomach was low, 0.8 % (60 min post injection), indicating that 99mTc-vinc was stable inside the body. The 99mTc-vinc was rapidly distributed after intravenous injection as shown by the renal elimination, although liver and spleen uptake was significant. Vincristine is cleared through hepatobiliary pathway with varying degree of renal extraction and liver retention like all 99mTc-iminodiacetic acid complexes [34]. The retention of 99mTc-vinc in liver may be attributed to its metabolism in liver. The study shows that uptake of a radiotracer is dependent on several factors, such as the nature of the radiotracer, blood flow and pH indicating a slow transfer of charged metabolites formed across the cell membrane. The two hydroxyl groups of vincristine play a crucial role in pharmacodynamics. After the transport inside the cell, the hydroxyl groups can be phosphorylated by cellular deoxyguanosine kinase [35]. Vincristine is a very important commercially available clinical medicine with known pharmacokinetics, pharmacodynamics and toxicity. The large activity of 99mTc-vinc in spleen suggests that 99mTc-vinc can target highly proliferating cells. The whole body SPECT imaging results agree well with in vivo biodistribution studies of 99mTc-vinc. The scan showed a high activity in liver, spleen and normal distribution in the whole body after 1 h administration of the 99mTc-vinc in mice and rabbits as depicted in Figs. 10a and 11a respectively. The activity was gradually increased in liver and slightly decreased in spleen with the passage of time (Fig. 10b, c). The scintigraphic images thus suggest that 99mTc-vinc possesses excellent characteristics for promising application as a novel splenic imaging agent in mice and rabbits. The high hydrophilic character of 99mTc-vinc is in accordance with its predominant renal clearance.
Conclusion
In present work 99mTc-vinc was designed, synthesized and evaluated biologically. Radiolabeling efficiency of 99mTc-vinc monitored by thin layer chromatography was 99.6 ± 0.4, (n = 5). Neutral charge on complex was determined by electrophoresis while HPLC results showed single species. Biological distribution and scintigraphic studies in normal mice and rabbit indicated higher accumulation of 99mTc-vinc in liver and spleen. Due to the attractive biological properties, 99mTc-vinc may serve as functional agent for diagnostic purposes.
References
Becker W (1995) The contribution of nuclear medicine to the patient with infection. Eur J Nucl Med 10:1195–1211
Oliva MR, Saini S (2004) Liver cancer imaging: role of CT, MRI, US and PET. Cancer Imaging. doi:10.1102/1470-7330.2004.0011
El-Ghany EA, Amine AM, El-Sayed AS, El-Kolaly MT, Abdel-Gelil F (2005) Radiochemical and biological characteristics of 99mTc-piroxicam for scintigraphy of inflammatory lesions. J Radioanal Nucl Chem 266(1):125–130
Hina S, Rajoka MI, Roohi S, Haque A, Qasim M (2014) Preparation, biodistribution, and scintigraphic evaluation of 99mTc-clindamycin: an infection imaging agent. Appl Biochem Biotechnol 174:1420–1433
Zahoor R, Roohi S, Ahmad M, Iqbal Z, Amir N, Tariq S, Savage PB (2013) Synthesis of 99mTc-cationic steroid antimicrobial-107 and in vitro evaluation. J Radioanal Nucl Chem 295:841–844
Amir N, Roohi S, Pervez S, Mushtaq A, Jehangir M, Miyashita Y, Okamoto K (2009) S-bridged complex of 99mTc with fac (S)-[Rh(aet)3]. Quality control, characterization and biodistribution studies in rats. J Radioanal Nucl Chem 279(1):25–30
Qaiser SS, Khan MR (2013) Synthesis and biological evaluation of the 99mTc-gemifloxacin dithiocarbamate complex: a novel Streptococcus pneumoniae infection imaging agent. J Mol Imaging Dyn 2(2):1–4
Faheem AR, Bokhari TH, Roohi S, Mushtaq A, Sohaib M (2013) 99mTc-Daunorubicin a potential brain imaging and theranostic agent: synthesis, quality control, characterization, biodistribution and scintigraphy. Nucl Med Biol 40:148–152
Faheem AR, Bokhari TH, Roohi S, Chem M (2012) A direct labeling of doxorubicin with technetium-99m: its optimization, characterization and quality control. J Radioanal Nucl 293:303–307
Altiparmak B, Lambrecht FY, Bayrak E, Durkan K (2010) Design and synthesis of 99m Tc-citro-folate for use as a tumor-targeted radiopharmaceutical. J Pharm 400:8–14
Sakr TM, Essa BM, El-Essawy FA, El-Mohty AA (2014) Synthesis and biodistribution of 99m Tc-PyDA as a potential marker for tumor hypoxia imaging. Radiochemistry 56:76–80
Zhang Y, Stevenson GD, Barber C, Furenlid LR, Barrett HH, Woolfenden JM, Zhao M, Liu Z (2013) Imaging of rat cerebral ischemia-reperfusion injury using 99mTc-labeled duramycin. Nucl Med Biol 40:80–88
Xu YP, Luo SN, Pan DH, Wang LZ, Zhou YR, Yang M (2013) Synthesis and preliminary evaluation of 99mTc-spermine as a tumor imaging agent. J Radioanal Nucl Chem 295:1861–1866
Amin AM, Sanad MH, Abd-Elhaliem SM (2013) Radiochemical and biological characterization of 99m Tc-piracetam for brain imaging. Radiochemistry 55(6):624–628
Gelfand M, Parisi M, Treves S (2011) Pediatric radiopharmaceutical administered doses: 2010 North American consensus guidelines. J Nucl Med 52:318–322
Norenberg JP, Hladik WB, Henkin RE (2006) Nuclear medicine, 2nd edn. Mosby Elsevier, Philadelphia, pp 938–948
Bartholoma MD, Louie AS, Valliant ZF, Zubieta J (2010) Technetium and gallium derived radiopharmaceuticals: comparing and contrasting the chemistry of two important radiometals for the molecular imaging era. Chem Rev 110:2903–2920
Sanad MH, El-Tawoosy M (2013) Labeling of ursodeoxycholic acid with technetium-99m for hepatobiliary imaging. J Radioanal Nucl Chem 298:1105–1109
Blaha V, Cihak I, Nicek F (1993) Clearance and distribution parameters of 99mTc-EHIDA, -DTPA and -MAG-3 by dynamic liver/kidney scintigraphy. Nucl Med Biol 20:89–93
Kula M, Karacavus S, Baskol M, Deniz K, Abdulrezzak U, Tutus A (2010) Hepatobiliary function assessed by 99mTc-mebrofenin cholescintigraphy in the evaluation of fibrosis in chronic hepatitis: histopathological correlation. Nucl Med Commun 31:280–285
Malhi H, Bhargava KK, Afriyie MO, Volenberg I, Schilsky LM, Palestro CJ, Gupta S (2002) 99mTc-mebrofenin scintigraphy for evaluating liver disease in a rat model of Wilson’s disease. J Nucl Med 43(2):246–252
Shen S, Jacob R, Bender LW, Duan J, Spencer SA (2014) A technique using 99mTc-mebrofenin SPECT for radiotherapy treatment planning for liver cancers or metastases. Med Dosim 39:7–11
Shah I, Bhatnagar S, Rangarajan V, Patankar N (2012) Utility of Tc-99m-mebrofenin hepato-biliary scintigraphy (HIDA scan) for the diagnosis of biliary atresia. Trop Gastroenterol 33:62–64
Lan JA, Chervu LR, Johansen KL, Wolkoff AW (1988) Uptake of technetium 99m hepatobiliary imaging agents by cultured rat hepatocytes. Gastroenterology 95:1625–1631
Ayoub SM, Abu Taleb AM, Ebeid NH (2014) Radiolabeling of alpha-fetoprotein monoclonal antibody for detection of liver tumor. Radiochemistry 56:81–85
Xiangting C, Qiaoyu L, Wenyu S, Feng Z, Xuehao W, Hai W (2012) Bromocriptine enhances the uptake of 99mTc-MIBI in patients with hepatocellular carcinoma. J Biomed Res 26:165–169
de Barros ALB, das Grac¸as Mota L, de Aguiar Ferreira C, Correˆa NCR, de Go´es AM, Oliveira MC, Cardoso VN (2013) 99m Tc-labeled bombesin analog for breast cancer identification. J Radioanal Nucl Chem 295:2083–2090
Oates E, Austin JM, Becker JL (1995) Technetium-99m-sulfur colloid SPECT imaging in infants with suspected heterotaxy syndrome. J Nucl Med 36:1368–1371
Pohlson EC, Wilkinson RW, Witzum KF (1994) Heat damaged red cell scan for intraoperative localization of the accessory spleen. J Pediatr Surg 29:604–608
Johnson IS, Armstrong JG, Gorman M, Burnett JP (1963) The vinca alkaloids: a new class of oncolytic agents. Cancer Res 23:1390–1427
Qweider M, Gilsbach JM, Rohde V (2007) Inadvertent intrathecal vincristine administration: a neurosurgical emergency: case report. J Neurosurg 6:280–283
Graf WD, Chance PF, Lensch MW, Eng LJ, Lipe HP, Bird TD (1996) Severe vincristine neuropathy in charcot-marie-tooth disease type 1A. Cancer 7:1356–1362
Chen X, Li L, Liu F, Liu B (2006) Synthesis and biological evaluation of technetium-99m-labeled deoxyglucose derivatives as imaging agents for tumor. Bioorg Med Chem Lett 16:5503–5506
Saha GB (2003) Fundamentals of nuclear pharmacy, 5th edn. Springer, New York
Colledge D, Civitico G (2000) In vitro antihepadnaviral activities of combinations of penciclovir, lamivudine, and adefovir. Antimicrob Agents Chemother 44(3):551–560
Acknowledgments
The authors are thankful to Mr. Ibrar Haider, Zafar, Riaz and Jamshed for their helpful discussions and providing facilities to work properly in lab.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hina, S., Roohi, S., Rajoka, M.I. et al. Preparation, quality control and biological characterization of 99mTc-vincristine. J Radioanal Nucl Chem 304, 553–561 (2015). https://doi.org/10.1007/s10967-014-3836-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3836-0