Abstract
This work reports the adsorption of strontium(II) from aqueous solutions onto calcium alginate (CA) and chitosan (CH) materials. The adsorption process was studied in batch experiments as a function of the pH of the solution, contact time, and temperature. Freundlich isotherm was found to describe the sorption process comparatively well as the Langmuir model. Laboratory obtained spherical beads of CA seems to be a better sorbent than commercially available materials. A contact time of about 4 h and neutral pH of the initial aqueous solution seem to be optimal conditions for Sr-85 to be removed from contaminated solutions using alginate beads.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
One of the most hazardous radionuclides present in low- and medium-level radioactive wastes (LLW and MLW, respectively) is strontium-90 (Sr-90; t 1/2 28.9 years; E β 546 keV). Its danger is caused by the long biological half-life of Sr-90 (49.3 years) and the element’s chemical similarity to calcium, which implies that it is capable accumulate in bone tissues. In addition, Sr-89 (t 1/2 50.6 days; E β 1,495 keV), which in the form of chloride appears to be useful in nuclear medicine for the treatment of bone cancer, and certain compounds of Sr-85 (t 1/2 64.8 days; E γ 514 keV), used in nuclear medicine as bone imaging agents, may be found in LLW and MLW [1–3].
Different techniques have been used to remove both radionuclides of strontium from aqueous samples and water streams, such as ion-exchange [4, 5], reverse osmosis [6], precipitation [7–9], or electrochemical treatment [10]. However, their routine application is limited both by the high operational cost and production of great amounts of slurries.
Among the main alternative methods, the accumulation of strontium onto inexpensive solid materials of biological origin should be mentioned [11–16]. The carbohydrate polymers: calcium alginate (CA) and chitosan (CH) are two of the most popular biosorbents (Fig. 1).
The aim of the present study was to examine both of the aforementioned biopolymers’ usefulness in the removal of strontium radionuclides from aqueous waste solutions. In particular, two types of CA and CH were studied. Sr-85 radionuclide was applied because of the excellent energy of emitted gamma radiation, which implies easy determination of the radionuclide content in the analyzed solutions. This radionuclide is often used to study the condition of bones in the human bodies.
Experimental
Chemicals
Sodium alginate and alginic acid calcium salt (purum), both powders, came from SIGMA-ALDRICH. Chitosan (molecular weight 100,000; acetylated form) was purchased from ACROS ORGANICS. All inorganic chemicals used in the experiments were purchased from SIGMA-ALDRICH. The solutions were prepared in deionized water with electrical conductivity lower than 10 μS cm−1 at 25 °C.
A carrier-free radionuclide of Sr-85 was supplied from Radioisotope Centre POLATOM, National Centre for Nuclear Research (Świerk, Poland).
CA spherical beads were prepared by dropping 0.5 M of CaCl2 aqueous solution into a 4 % w/w solution of sodium alginate in water with continuously stirring it at room temperature. The final solution was left to stand for additional 24 h. Grains of the sorbent (ca. 3 mm in the diameter) were stored in a solution containing 0.01 M of KCl and 0.001 M of CaCl2. Before application, CA beads were rinsed carefully with a large amount of the deionized water.
Sorption experiments
The sorption of Sr(II) was studied as a function of the contact time, initial pH, and temperature in separate experiments. About 0.1 g of each sorbent was added to 2.0 mL of strontium aqueous solution (in polyethylene vials) and shaken in the thermostatic shaker at a temperature of 10, 25, or 50 °C respectively. In all experiments, except those of time dependence, solid sorbents had contact with the liquid phase for 4 h. The initial concentration of Sr(II) radionuclide in the solutions was kept ~100 kBq mL−1 and has been determined radiometrically, simultaneously with the equilibrium concentrations.
Because of the low Sr(II) initial concentration, it is practical to present the results in terms of the distribution coefficient (K d):
where C 2, C 1, and C 0 are the concentrations (kBq mL−1) of Sr(II) on the sorbent, residual Sr(II) concentration in the equilibrium solution, and in the initial bulk solution, respectively. Factor V is the volume of the aqueous phase (mL) and W is weight of the sorbent (g).
This term describes the ratio of the total analytical concentration of a substance in the sorbent to its total analytical contents in the aqueous phase in equilibrium [17].
In each experiment, the mean value of K d was estimated from triplicate measurements.
Sorption isotherms
Studies were carried out in the batch mode experiments as described above. A series of experiments was carried out with aqueous phases containing well-defined initial concentrations of the radionuclide in the nca scale. The range of the concentrations was expressed in kBqs and raised from ~53 up to ~425 kBq mL−1. Initial and equilibrium samples of Sr-85 were analyzed in an automatic well-type gamma ray detector (E γ = 514 keV) after its calibration in a gamma radiation calibrator.
The linearized form of the equations used were:
where q is the equilibrium metal uptake capacity and C e the residual metal concentration at equilibrium. The constant k is a measure of the adsorption capacity and 1/n is the intensity of adsorption.
The constant q max represents the maximum specific metal uptake and b is the ratio of the adsorption/desorption rates related the adsorption energy through the Arrhenius equation.
Chemical equilibrium diagrams
The calculations done by Hydra-Medusa Software (Make Equilibrium Diagrams Using Sophisticated Algorithms [18] ) were performed to create chemical equilibrium diagrams.
Results and discussion
Kinetics of sorption
Figure 2 shows the time profiles of the strontium(II) sorption onto both studied types of CA and (CH). As it can be seen, adsorption of Sr(II) on all examined sorbents was quick and the equilibrium was reached already after 1 h. After this time sorption is independent from the contact time of equilibrated phases. In detail, the decontamination factors (DF = radiation activity before sorption/radiation level after sorption) at a pH of 5.6 and a temperature of 25 °C are: 139 ± 15 (commercial alginate), 3.91 ± 0.77 (alginate beads), and 36.6 ± 4.9 (chitosan), respectively. Based on these results, in further experiments it was chosen to contact solid sorbents with the liquid phase for 4–6 h.
Effect of temperature
In order to evaluate the effect of the temperature on strontium removal, thermodynamic parameters such as enthalpy (ΔH 0), entropy (ΔS 0), and Gibbs free energy (ΔG 0) were investigated. Therefore, the sorption of Sr(II) on all sorbents at 10, 25, and 50 °C was studied, and results are shown in Fig. 3.
To estimate the thermodynamic functions, the relationship was used [19].
where terms ΔG 0, ΔH 0 and ΔS 0 have the meaning shown above. T stands for the temperature (°K) and R for the gas constant (8.314 J mol−1 K−1). The linear representation of log K d as a function of temperature provides the values of enthalpy and entropy change from the slope and intercept of the plots (see Fig. 3). Their values are tabulated in Table 1 and contain terms due to temperature changes in the activity coefficients of the species involved. Assuming that these changes are similar for the solutions of Sr(II) in all of the systems studied, the enthalpy and entropy of sorption can be compared.
As seen in Fig. 3, the plots of logK d in the function of temperature are linear. Changes in the standard free energy increase with increasing temperatures regardless of the type of sorbent. This indicates that better adsorption is actually obtained at higher temperatures. The free energy of the process at all temperatures is negative and changes with the rise in temperature. The negative values of ΔG 0 at all temperatures studied are due to the fact that the adsorption process is spontaneous (does not require the delivery of the energy). The positive value of ΔS 0 suggests increased randomness at the solid and solution interface during the adsorption of metal ions onto adsorbent [20]. The negative values of ΔH 0 indicate the exothermic nature of the adsorption process.
Effect of initial pH
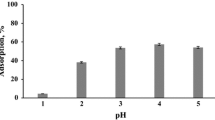
The results obtained for strontium(II) sorption for the initial pH ranging from 3 to 9 are illustrated in Fig. 4.
As seen from the Fig. 4, sorption of strontium(II) on all sorbents strongly depends on the initial pH of the solution and raises with an increasing pH as high as 8–9. Furthermore, the distribution coefficients for commercial CA exceed these for CH and CA beads.
The sorption of any metal ion as a function of the initial pH, as a rule, is a complex process. Different forms of metal present in the solution, hydrolyzed and complexed, should also be taken into account. Different forms of the sorbent surface may influence the process as well.
The chemical equilibrium calculation shows that content of the Sr(II) species remains nearly constant in the pH region of 1–9 (Fig. 5). SrHCO3 + and SrCO3 (formed with carbon dioxide present in the air) start to appear at pH of above 8.5. These species are not highly soluble in water (e.g., SrCO3 ca 4 × 10−5 mol L−1 [21]), so their appearance in solutions of pH higher than 8 containing microamounts of Sr(II) should be taken into account.
On the other hand, the ionization state of functional groups present in the surface region of the sorbents is also highly dependent on pH. It is known from the literature that carboxyl groups in biomass play an important role in metal sequestration, and they are responsible for a major fraction of metal immobilization [11]. The pK a value of a carboxylic group existing in biomass varies from about 2 to 4.7 [11]; therefore, at a pH of around 4.5, the extent of its dissociation should roughly approach 50 %. Besides, in acidic solutions the majority of amine surface groups in CH may be protonated, inducing less of a negative charge than in the basic conditionsFootnote 1; hence, in acidic solutions, their interactions with positively charged strontium(II) species are not favored by electrostatic forces.
Sorption isotherms
The adsorption isotherm equation is a description of the processes governing the sorption of a substance from the aqueous environment to a solid phase at well-defined conditions (temperature and pH). The parameters of these equations express the surface properties of the sorbent and its affinity toward the sorbed species. Thus, such a mathematical description is necessary for the design of future sorption systems. Over the years, a wide variety of equilibrium isotherm models have been formulated. All of them are listed and discussed in paper [23], which provides also their linear forms and coordinates used for the diagrams’ construction.
Until now, for the two parameter isotherms (e.g. Langmuir or Freundlich), the accuracy of the fit of an isotherm to experimental equilibrium data has been based on the magnitude of the coefficient of determination for the linear regression. This means that the isotherm giving an R 2 value closest to unity was believed to provide the best fit.
The sorption data were analyzed according to the linear forms of the Langmuir and Freundlich models. Illustration of the plots is shown in Fig. 6 and the linear isotherm constants - b, q m, log(K f) and 1/n f - are presented in Table 2. All isotherms were found to be linear over the whole range of studied concentrations. The R 2 values compared for both models applied for describtion of each system suggest that both isotherms offer equally good modelling. So, it may be justified to conclude that both physical and chemical mechanisms of strontium(II) sorption have thair contribution to the overall process in the nca scale.
Evaluation of the thermal stability of sorbents
The thermogravimetric (TGA) data (Fig. 7) for all studied sorbents showed a sharp reduction in weight at the temperatures lower than ~300 °C. However, thermal decomposition of spherical CA beads occurs at much lower temperatures than of the as-received sorbents. The samples’ weight loss to 25 wt% was detected at 156, 545, and 762 °C for the CA beads, commercial chitosan, and commercial CA, respectively. It should be noted that for the sodium alginate (being the substrate), the sample weight to 25 wt% occurs at about 680 °C [24]. The total weight loss curves for both as-received sorbents show three sharply decreasing parts, which may be attributed to the liberation of the adsorbed water and decomposition of the sorbents [25]. It seems that synthesized spherical CA beads at the same time loose water and decompose to the CaCO3 in the temperature below 200 °C.
Conclusions
Laboratory obtained beads of CA seem to be a better sorbent than commercially available materials. They are the most effective in strontium(II) adsorption potentially present in aqueous solutions and decompose at lower temperatures giving the opportunity to burn at low temperature of sorbed wastes and by this lower their volume for further long-term disposal. The TGA properties of this material show that pretreatment of the radioactive wastes containing strontium immobilized on the spherical CA beads requires less energy input than on the as-received sorbents.
A contact time of about 4 h and neutral pH of the initial aqueous solution seems to be the optimum conditions for Sr-85 to be removed from contaminated solutions using alginate beads.
Notes
Appropriate theoretical calculations were carried out by using the SPARTAN 2008 program [22] running on the PC. Geometry optimization and calculation of the atomic charges in the d-glucosamine and N-acetyl-d-glucosamine were performed at the Hartree–Fock level within the model UHF/3-21G(*). In particular, Natural Atomic Charges of the amine group decrease from −0.9246 and −0.7600 to −0.2870 and −0.3280 in the d-glucosamine and N-acetyl-d-glucosamine, respectively.
References
Saha GB (2004) Fundamentals of nuclear pharmacy. 5th ed. Springer, New York, pp 60, 130, 346
Nightengale B, Brune M, Blizzard SP, Ashley-Johnson M, Slan S (1995) Strontium chloride Sr 89 for treating pain from metastatic bone disease. Am J Health Syst Pharm 52:2189–2195
Robinson RG, Preston DF, Schiefelbein M, Baxter KG (1995) Strontium-89 therapy for the palliation of pain due to osseous metastases. J Am Med Assoc 274:420–424
Amano H, Yanase N (1990) Measurement of 90Sr in environmental samples by cation-exchange and liquid scintillation counting. Talanta 37:585–590
Stella R, Valentini MTG, Maggi L (1993) Determination of 90Sr and milk by using two inorganic exchangers. Appl Radiat Isot 44:1093–1096
Wüthrich M, Mauch H (1975) Wasseraufbereitung durch umgekehrte osmose. [Water Treatment by Reversed Osmosis.]. Tech Mitt PTT, 53:252–263
Benzi P, Operti L, Volpe P (1988) On the reliability of a rapid method for the determination of 90Sr in natural samples. J Radioanal Nucl Chem 126:245–256
Bojanowski R, Knapinska-Skiba D (1990) Determination of low-level 90Sr in environmental materials: a novel approach to the classical method. J Radioanal Nucl Chem 138:207–218
Blackbum R, Al-Masri MS (1993) Radioassay of strontium-90 in the presence of calcium-45 and radiocaesium (134Cs and 137Cs). Appl Radiat Isot 44:683–686
Rudd EJ, Walton CW (eds) (2000) Environmental aspects of electrochemical technology: radiological decontamination. The Electrochemical Society, Pennington
Naja GM, Volesky B (2009) Treatment of metal-bearing effluents: removal and recovery. Taylor & Francis and CRC Press, Boca Raton
Mimura H, Ohta H, Akiba K, Onodera Y (2001) Uptake behaviour of americium on alginic acid and alginate polymer gels. J Radioanal Nucl Chem 247:33–38
Dabbagh R, Ghafourian H, Baghvand A, Nabi GR, Ahmadi Faghih MA, Riahi H (2007) Bioaccumulation and biosorption of stable strontium and 90Sr by Oscillatoria homogenea cyanobacterium. J Radioanal Nucl Chem 272:53–59
Barot NS, Bagla HK (2012) Biosorption of radiotoxic 90Sr by green adsorbent: dry cow dung pow-der. J Radioanal Nucl Chem 294:81–86
Gok C, Gerstmann U, Aytas S (2013) Biosorption of radiostrontium by alginate beads. Application of isotherm models and thermodynamic studies. J Radioanal Nucl Chem 295:777–788
Imessaoudene D, Bouzidi A, Hanini S (2013) Biosorption of strontium from aqueous solutions onto spent coffee grounds. J Radioanal Nucl Chem 298:893–902
Freiser H, Nancollas GA (1987) Compendium of analytical nomenclature, 2nd edn, chap 3. Definitive rules 1987 IUPAC. Blackwell Scientific Publications, London
Chemical equilibrium diagrams: https://sites.google.com/site/chemdiagr/2013
Fuks L, Fidelis I (1987) Thermodynamic studies of complex formation of actinyl ions extracted with TBP from hydrochloric and nitric acids. J Radioanal Nucl Chem Lett 118:361–368
Aksu Z (2002) Determination of the equilibrium, kinetic and thermodynamic parameters of the batch biosorption of nickel(II) ions ontoChlorella vulgaris. Process Biochem 38:89–99
Townley RR, Whitney WB, Felsing WA (1937) The solubilities of barium and strontium car-bonates in aqueous solutions of some alkali chlorides. J Am Chem Soc 59:631–633
’08, Wavefunction Inc., Irvine CA, USA, 2006-2009; ISBN978-1-890661-38-4
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10
Cheong M, Zhitomirsky I (2008) Electrodeposition of alginic acid and composite films. Colloids Surf A: Physicochem Eng Asp 328:73–78
Soares JP, Santos JE, Chierice GO, Cavalheiro ETG (2004) Thermal behavior of alginic acid and its sodium salt. Ecl Quim 29:57–63
Acknowledgments
This research has been financed from the National Centre for Research and Development (Poland) through the Strategic Program "Technologies Supporting Development of Safe Nuclear Power Engineering", task 4 "Development of spent nuclear fuel and radioactive waste management techniques and technologies".
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fuks, L., Oszczak, A., Gniazdowska, E. et al. Calcium alginate and chitosan as potential sorbents for strontium radionuclide. J Radioanal Nucl Chem 304, 15–20 (2015). https://doi.org/10.1007/s10967-014-3698-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3698-5