Abstract
The silica/polymer-based isoHex-BTP adsorbent (isoHex-BTP/SiO2-P) was prepared for partitioning Am(III) and Cm(III) from HLLW. Batch adsorption results showed that isoHex-BTP/SiO2-P exhibited high affinity and selectivity for Am(III) and Pu(IV) over U(VI), Ln(III) and other typical fission products in 3 mol dm−3 nitric acid. The effects of contact time, initial Dy(III) concentration and temperature on the adsorption of Dy(III) [as a simulated element of MA(III) and a representative element of Ln(III)] by isoHex-BTP/SiO2-P in 3 mol dm−3 nitric acid were investigated. The adsorption of Dy(III) fitted well to the pseudo-second-order kinetic model and the Langmuir isotherm model. The adsorption thermodynamic parameters revealed that the adsorption was a spontaneous, endothermic and entropy-driving process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The so-called minor actinides, americium, curium and neptunium, as well as a small amount of residual plutonium are the most important long-lived radionuclides, which are responsible for the long-term radiation hazards of vitrified high level liquid waste (HLLW) arising from the PUREX process [1–3]. Separating and transmuting them to short-lived or stable nuclides prior to the vitrification step could have a significant beneficial impact on bringing down the surveillance period from millions of years to a few hundred years. The selective separation of trivalent minor actinides [MA(III): Am(III) and Cm(III)] from the trivalent lanthanides (Ln(III)) which are present being about 20 up to 50 times more abundant than MA(III) in HLLW [3, 4] is important in P & T (Partitioning and Transmutation) strategy since some of the lanthanides exhibit large neutron-capture cross-sections, hence reducing transmutation efficiency of the minor actinides [5]. However, MA(III)-Ln(III)-separation is a challenging task that remains to be solved owing to their similar coordination chemistry and their disadvantageous ratio of presence in HLLW [4, 6, 7]. Currently, several two-step partitioning processes based on liquid–liquid extraction have been designed: the minor actinides and lanthanides are firstly co-extracted from the bulk of the fission products using oxygen donor ligands, e.g. CMPO [8], DMDOHEMA [9], TRPO [10] and DIDPA [11] in TRUEX [12], DIAMEX [12], TRPO [10] and DIDPA [11] processes, and subsequently the minor actinides could be selectively separated from the lanthanides using nitrogen or sulfur donor ligands in SANEX [1, 13, 14], CYANEX 301 [15, 16] processes, etc. Among various nitrogen or sulfur donor ligands, 2,6-bis(5,6-dialkyl-1,2,4-triazin-3-yl) pyridines (known as BTPs) turn out to be highly selective extractants for MA(III) over Ln(III) from highly acidic solutions (HNO3 > 1 mol dm−3) [17]. They fulfill CHON principle, i.e., the species consist entirely of carbon, hydrogen, oxygen, and nitrogen atoms to make them combustible to safe gaseous products [18, 19]. So, many researches predominantly in Europe have been focused on the design of nitrogen-donor ligands for MA(III) separation [1, 7, 20]. However, a potential process application requires selectivity, fast kinetics, stability, high solubility and loading capacity [5]. The extracting agents BTPs available so far suffer from one or more drawbacks in liquid–liquid extraction system [5, 21], which might result in generating a great amount of organic waste and requiring extensive equipment for the multi-stage extraction, stripping and solvent-washing processes [22]. This requires further optimization to comply with process constrains. Therefore, a series of BTP derivatives where C–H bonds in the α-positions of the triazinyl rings are replaced by C–C bonds have been developed to increase the stability of this kind of ligands against hydrolysis and radiolysis as well as to improve the extracted capacity and the strip performance of MA(III) [5, 6]. On the other hand, these drawbacks can be alleviated by use of alternative techniques such as extraction chromatography and liquid membrane method [22–24]. Extraction chromatographic technique combines the selectivity of liquid–liquid extraction with the ease of operation of column chromatography for the clean separation of MA(III) [15, 25–27]. Furthermore, the feed for the PUREX process produced by dissolving commercial spent fuel (45.2 GWd tM−1) in 4 mol dm−3 HNO3 contained 131,917 μg g−1 U, 1,153 μg g−1 Pu, 24 μg g−1 243Am and 6 μg g−1 244Cm [3], therefore, compared to uranium and plutonium, the minor actinides are significantly less abundant in the spent fuel indicating that the scale of the separation process for minor actinides from HLLW should be considerably smaller than that of a main separation process such as PUREX [28, 29].
As an alternative technology of the liquid–liquid extraction process, we have reported a direct separation process of MA(III) from HLLW based on extraction chromatography (Fig. 1) [29, 30]. The process uses a single-column packed with silica/polymer-based BTP adsorbent named as BTP/SiO2-P, e.g. isoHex-BTP/SiO2-P [isoHex-BTP: 2,6-bis(5,6-diisohexyl)-1,2,4-triazin-3-yl)pyridine]. The extraction chromatographic material, isoHex-BTP/SiO2-P adsorbent, using silica/polymer composite support (SiO2-P) as the inert support and isoHex-BTP as the extracting agent, was prepared. SiO2-P support contains a macroreticular styrene–divinylbenzene copolymer which is immobilized in porous silica particles with pore size of 0.6 μm and mean diameter of 60 μm. The content of extracting agent isoHex-BTP in isoHex-BTP/SiO2-P adsorbent was relatively high (up to 33.3 % of the total mass of the adsorbent). So the adsorbent may overcome the limitation of the low solubility of BTPs in traditional aliphatic diluents [23, 26]. Compared to liquid–liquid extraction method, the main advantages of the partitioning process include not only its high adsorption selectivity and affinity towards Am(III) over Ln(III) and other fission products present in HLLW, but also much smaller scale equipment than that of PUREX process, savings in reagent and waste disposal costs, possibility of direct separation of MA(III) in the column mode [24, 29, 30].
In the present work, to further study the properties of isoHex-BTP/SiO2-P, the adsorption behavior of 241Am(III), 239Pu(IV), 238U(VI), Ln(III) and other typical fission products by isoHex-BTP/SiO2-P adsorbent from nitric acid solution was investigated. The effects of various parameters including contact time, initial concentration of Dy(III) and adsorption temperature on the adsorption of Dy(III) [as a simulated element of MA(III) and a representative element of Ln(III)] by isoHex-BTP/SiO2-P from 3 mol dm−3 nitric acid solution were studied. The experimental adsorption data depending on contact time were explained by the pseudo-first-order and pseudo-second-order kinetic models. The equilibrium adsorption data were analyzed using the Freundlich and Langmuir isotherm models. Thermodynamic parameters, Gibbs free energy change (∆G), enthalpy change (∆H) and entropy change (∆S), were calculated by using adsorption data changing on temperature to evaluate the thermodynamic feasibility and the spontaneous nature of the adsorption process.
Experimental
Materials and characterization
Various reagents, RE(NO3)3·nH2O (RE: Y, La, Ce, Nd, Sm, Eu, Gd and Dy, n = 5 or 6), other fission product (FP) element nitrates [Sr(II) and Zr(IV)] and (NH4)6Mo7O24·4H2O, were of commercial reagents of analytical grade. The solutions of these metal ions were prepared by dissolving the reagents into nitric acid solutions. Ru(III) solution was obtained by diluting a commercially available nitrosyl nitrate solution containing 1.5 % of Ru(III). 1 mmol dm−3 of U(VI), trace amount of 241Am(III), 152Eu(III), 99Tc(VII) and 239Pu(IV) were prepared from their stocked solutions, respectively.
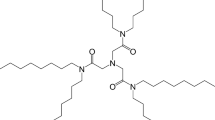
The purity of synthesized extracting agent, isoHex-BTP, was 99 %. isoHex-BTP/SiO2-P adsorbent was prepared as described in the previous studies of Wei et al. [31–34] by impregnating isoHex-BTP molecules into the pores of the silica/polymer composite support (SiO2-P). isoHex-BTP/SiO2-P contained 0.5 g of isoHex-BTP in 1.0 g of SiO2-P, i.e. the content of extracting agent isoHex-BTP was as high as 33.3 % of the total mass of the adsorbent. The chemical structure of isoHex-BTP and scanning electron microscope (SEM) image of isoHex-BTP/SiO2-P are shown in Fig. 2 [29, 34].
Batch adsorption experiment
The adsorption behaviors of 241Am(III) (1,000 Bq cm−3), 239Pu(IV) (50 Bq cm−3), 238U(VI) (1 mmol dm−3), 152Eu(III) (1,000 Bq cm−3), 99Tc(VII) (1,000 Bq cm−3) and other typical fission products by isoHex-BTP/SiO2-P adsorbent from nitric acid medium were studied in a batch adsorption mode. The effects of adsorption parameters including contact time (10 min–72 h), initial Dy(III) concentration and adsorption temperature (288–308 K) on the adsorption of Dy(III) by isoHex-BTP/SiO2-P from 3 mol dm−3 nitric acid solution were studied in the batch adsorption mode, too.
For each batch experiment, 5 cm3 of aqueous solution containing metal ions and nitric acid was added to 0.1 g isoHex-BTP/SiO2-P in a glass vial with Teflon stopper. The mixture was shaken mechanically at 120 rpm for predetermined contact time in a constant temperature water bath. Then the aqueous phase was filtrated through a membrane filter with 0.20 μm pore. The concentration of individual metal ions such as Sr(II), Y(III), Zr(IV), Mo(VI), Ru(III), La(III), Ce(III), Nd(III), Sm(III), Eu(III), Gd(III) and Dy(III) present in aqueous phase before and after the adsorption was analyzed by Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES: Shimadzu ICPS-7510). The individual radioactivity of 241Am and 152Eu was determined by HPGe-γ-detector (GEM70P-PLUS, Ortec). The individual radioactivity of 99Tc(VII) and 239Pu(IV) was measured by Super Low Level Liquid Scintillation Analyzer (PE Tri-Carb 3170). The concentration of U(VI) was measured by UV spectrophotometer (LabTech UV1000/1100).
In a batch experiment, the distribution coefficient (K d, cm3 g−1) was calculated by Eq. (1) or Eq. (2):
The equilibrium adsorption capacity, q e (mmol g−1), and the adsorption capacity at the time t, q t (mmol g−1), were obtained using Eqs. (3) and (4), respectively:
where C 0 (mmol dm−3) [A 0 (Bq cm–3)] and C e (mmol dm−3) [A e (Bq cm−3)] are the concentration (radioactivity) of metal ions in the aqueous phase initially and at equilibrium, respectively. V (cm3) and W R (g) indicate the volume of the aqueous phase and the weight of isoHex-BTP/SiO2-P, respectively. C t (mmol dm−3) is the metal ion concentration in aqueous phase at a given contact time t.
Most of the experiments were repeated three times for better accuracy and blank experiments were also performed in this study. The experimental error was observed to be within ±5 %.
Experimental results
Selective adsorption of Am(III) and Pu(IV) by isoHex-BTP/SiO2-P
To study the impact of the initial nitric acid concentration on the adsorption of actinides and some typical fission products by isoHex-BTP/SiO2-P, 0.1 g isoHex-BTP/SiO2-P was batch equilibrated with 5 cm3 of solution containing 241Am(III) (1,000 Bq cm−3), 152Eu(III) (1,000 Bq cm−3), 99Tc(VII) (1,000 Bq cm−3), 239Pu(IV) (50 Bq cm−3), 1 mmol dm−3 238U(VI) and stable Eu(III) at various initial nitric acid concentrations for 24 h of contact. Figure 3 shows the plots of distribution coefficients, K d, of these nuclides with varying nitric acid concentration. Evidently, K d of Am(III) remarkably increased from 1 to 16,200 cm3 g−1 with increasing nitric acid concentration from 0.01 to 3 mol dm−3 owing to the participation of NO3 − in complexation reaction between Am(III) and isoHex-BTP/SiO2-P [29]. And then the slight decrease of K d of Am(III) from 3 mol dm−3 to higher concentration of nitric acid is thought to be due to a competition reaction of Am(III) or HNO3 with isoHex-BTP/SiO2-P [17]. While for Pu(IV), K d extraordinarily increased from 3 to 34,500 cm3 g−1 with increasing nitric acid concentration from 0.5 to 4 mol dm−3. Generally, both Am(III) and Pu(IV) were adsorbed efficiently from 2 to 4 mol dm−3 nitric acid solutions. However, the adsorption of 152Eu(III), 99Tc(VII) and U(VI) was very weak throughout the investigated nitric acid concentration range (0.01–4 mol dm−3). Although the distribution coefficients of trace amount of 152Eu(III) were higher than those of 1 mmol dm−3 stable Eu(III) in 2–4 mol dm−3 nitric acid solutions as shown in Fig. 3 and Table 1, they are still much lower than those of trace amount of Pu(IV) and Am(III). The order of adsorbability was Pu(IV) > Am(III) ≫ Eu(III), Tc(VII) and U(VI) within the nitric acid concentration range of 2–4 mol dm−3.
Effect of initial nitric acid concentration on the distribution coefficient of 241Am(III), 152Eu(III), 239Pu(IV), 99Tc(VII), 238U(VI) and stable Eu(III) (298 K, phase ratio: 0.1 g 5 cm−3, trace amount of 241Am(III), 152Eu(III), 99Tc(VII) and 239Pu(IV), 238U(VI) and stable Eu(III): 1 mmol dm−3, shaking speed: 120 rpm, contact time: 24 h)
Since isoHex-BTP/SiO2-P is a potential adsorbent in the direct separation process of MA (III) from HLLW by extraction chromatography in our research work, knowing its selectivity for Am(III) and Pu(IV) over Eu(III), U(VI) and Tc(VII) does not suffice. According to the nitric acid concentration of HLLW (about 3 mol dm−3) and the experimental data in Fig. 3, 3 mol dm−3 HNO3 solution was chosen to investigate the adsorption of actinides [i.e. 241Am(III), 239Pu(IV) and 238U(VI)] and some typical fission products by isoHex-BTP/SiO2-P for 24 h of contact at 298 K. The distribution coefficient of each element is illustrated in Fig. 4. It can be observed that Sr(II), Zr(IV), Mo(VI), Tc(VII), Ru(III), Y(III), all Ln(III) and U(VI) showed very weak or almost no adsorption towards isoHex-BTP/SiO2-P. Such low values in the distribution coefficient were due to the weak complexation of these metal ions with isoHex-BTP/SiO2-P. Contrastively, K d values of Pu(IV) (20,400 cm3 g−1) and Am(III) (16,200 cm3 g−1) were extremely high. The reason is that the radial extension of the 5f orbitals of Pu(IV) and Am(III) is further than the 4f orbitals of Ln(III) and outermost electron orbitals of other fission products, which causes bonds of Pu(IV) and Am(III) to have more covalent character and Pu(IV) and Am(III) forming more stable complexes with isoHex-BTP/SiO2-P [35]. The extremely high selectivity of Pu(IV) can be interpreted as the size fit of isoHex-BTP cavity being preferable for Pu(IV), which needs further researches [7, 17, 35]. The separation factors between 241Am(III) or 239Pu(IV) and the most affinity nuclide, Eu(III), among the other experimental metal ions, \( SF_{{{}^{ 2 4 1}{\text{Am/}}{}^{ 1 5 2}{\text{Eu}}}} \) (\( SF_{{{}^{ 2 4 1}{\text{Am/}}{}^{ 1 5 2}{\text{Eu}}}} = K_{{{\text{d}}\;{}^{ 2 4 1}{\text{Am}}}} /K_{\text{d}} {}_{{{}^{ 1 5 2}{\text{Eu}}}} \)) and \( SF_{{{}^{ 2 3 9}{\text{Pu/}}{}^{ 1 5 2}{\text{Eu}}}} \)(\( SF_{{{}^{ 2 3 9}{\text{Pu/}}{}^{ 1 5 2}{\text{Eu}}}} = K_{{{\text{d}}\;{}^{ 2 3 9}{\text{Pu}}}} /K_{\text{d}} {}_{{{}^{ 1 5 2}{\text{Eu}}}} \)), were 61 and 77, respectively. The separation factors between 241Am(III) or 239Pu(IV) and the other metal ions were far more than 100.
Adsorption of actinides, lanthanides (III) and other typical fission products by isoHex-BTP/SiO2-P (298 K, [HNO3]initial: 3 mol dm−3, phase ratio: 0.1 g 5 cm−3, trace amount of 241Am(III), 239Pu(IV), 99Tc(VII) and 152Eu(III), stable Eu(III) and other metal ion concentration: 1 mmol dm−3, shaking speed: 120 rpm, contact time: 24 h)
As described in our previous report [29], isoHex-BTP/SiO2-P exhibited high selectivity for Am(III) over Ln(III) and other typical fission products. These results in the present work further suggest that isoHex-BTP/SiO2-P has relatively high selectivity and affinity for Am(III) and Pu(IV) over U(VI), Tc(VII), Eu(III) and other typical fission products in 3 mol dm−3 nitric acid solution. And the adsorbed Am(III) and Pu(IV) can be stripped into dilute nitric acid (<0.1 mol dm−3) or distilled water because Am(III) and Pu(IV) showed almost no adsorption in ≤1 mol dm−3 nitric acid solutions and the adsorption of Am(III) and Pu(IV) is readily reversible by changing the nitric acid concentration (as shown in Fig. 3). So it is possible to direct separate Am(III) and residual Pu(IV) from other metal ions present in HLLW by extraction chromatography using isoHex-BTP/SiO2-P.
According to Fig. 4 and our previous work [29, 30], K d value of Dy(III) was dominantly higher than those of other fission products in 3 mol dm−3 HNO3 and the adsorption behavior of Dy(III) by BTP/SiO2-P adsorbent was similar to that of MA(III) owing to their similar coordination chemistry [6, 7]. Furthermore, Dy(III) is hardly contained in HLLW. So as a simulated element of MA(III) and a representative element of Ln(III), Dy(III) adsorption by isoHex-BTP/SiO2-P from 3 mol dm−3 nitric acid solution was investigated to evaluate its adsorption kinetics, isotherms and thermodynamics in this study.
Effect of contact time on Dy(III) adsorption
To determine the equilibrium time for the adsorption, Dy(III) (initial concentration: 6 mmol dm−3) adsorption by isoHex-BTP/SiO2-P from 3 mol dm−3 HNO3 solution was studied as a function of contact time at different temperatures (288, 298 and 308 K, respectively). The results are shown in Fig. 5. Generally, Dy(III) adsorption capacity increased with the increase in contact time and temperature. With increasing contact time, a rapid initial Dy(III) adsorption within the first 3 h of contact was followed by a steady stage which finally reached an apparent plateau until adsorption equilibrium was attained. 48.8, 79.6 and 88.4 % of the equilibrium adsorption capacity of Dy(III) occurred within the first 3 h at 288, 298 and 308 K, respectively. The sudden increase of q t at the very beginning of the process is attributed to an abundant availability of active sites on internal and external surface area of isoHex-BTP/SiO2-P. With the progressive occupancy of these sites by Dy(III), the process comes into a period of slower adsorption, during which the less accessible sites can be occupied by Dy(III). Furthermore, the experimental equilibrium adsorption capacity (q e,exp) of Dy(III) increased from 0.14 to 0.17 mmol g−1 when adsorption temperature increased from 288 to 308 K (as shown in Fig. 5 and Table 2).
Effect of initial Dy(III) concentration on adsorption capacity
The equilibrium adsorption capacity of Dy(III) by isoHex-BTP/SiO2-P is a function of both the initial Dy(III) concentration and the adsorption temperature. The effect of initial Dy(III) concentration ranging from 2 to 6 mmol dm−3 on the equilibrium adsorption capacity of Dy(III) for constant contact time (t = 72 h, to obtain the complete equilibrium state) at constant temperature (288, 298 and 308 K, respectively) is plotted in Fig. 6. Apparently, the equilibrium adsorption capacity of Dy(III) (q e) increased by increasing the initial concentration of Dy(III) and then reached the maximum adsorption capacity (q m) at high initial concentration. It was observed that at lower initial concentration of Dy(III), the equilibrium adsorption capacity increased with increasing the temperature from 288 to 308 K due to higher utilization of active sites of isoHex-BTP/SiO2-P at higher temperatures. However, the experimental maximum adsorption capacity, q m,exp, almost remained constant (q m,exp = 0.16, 0.17 and 0.17 mmol g−1 at 288, 298 and 308 K, respectively, as shown in Fig. 6 and Table 3) despite of increasing temperature because the total existing adsorption sites of isoHex-BTP/SiO2-P are confined, which resulted in a saturation adsorption of Dy(III) [36, 37].
Discussion
Adsorption kinetics
In order to examine the mechanism of adsorption potential rate controlling steps such as mass-transport, and chemical reaction processes of the adsorption of Dy(III) by isoHex-BTP/SiO2-P, the experimental data obtained in the “Effect of contact time on Dy(III) adsorption” were further analyzed using the pseudo-first-order and pseudo-second-order kinetic models.
The pseudo-first-order kinetic model is based on the approximation that the adsorption rate relates to the number of the unoccupied, adsorptive sites [38]. The linear form of the model can be expressed as Eq. (5) [38, 39]:
where q e (mmol g−1) and q t (mmol g−1) are the adsorption capacity at equilibrium and at any time t (h), and k 1 is the adsorption rate constant (h−1).
The value of the adsorption rate constant (k 1) can be calculated by plotting of ln(q e− q t) versus t. k 1 and the correlation coefficients (R 2) at various temperatures were determined as summarized in Table 2. R 2 was fairly low (R 2 = 0.983, 0.670 and 0.531 at 288, 298 and 308 K, respectively) and the value of k 1 decreased from 0.11 to 0.08 h−1 with increase in temperature from 288 to 308 K which conflicted with the experimental phenomenon, indicating that the adsorption process does not fit to the pseudo-first-order kinetic model.
Experimental data were also applied to the pseudo-second-order kinetic model which is based on the assumption that chemisorption is the rate-determining step of adsorption process [40]. The linear form of the model can be expressed as Eq. (6) [39, 40]:
where q t (mmol g−1) and q e (mmol g−1) are the adsorption capacity at any time t and at equilibrium, k 2 (g mmol−1 h−1) is the adsorption rate constant of pseudo-second-order kinetic model.
The adsorption rate constant k 2 and the calculated equilibrium capacity q e,calc can be calculated by plotting of t/q t versus t for Dy(III) adsorption by isoHex-BTP/SiO2-P. The kinetic parameters including k 2, q e,calc, h (initial adsorption rate, h = k 2 q 2e ) and R 2 (correlation coefficient) at various temperatures are listed in Table 2. As shown in Table 2, R 2 for the pseudo-second-order kinetic plot increased from 0.988 to 1.000 with increasing temperature from 288 to 308 K and q e,calc was almost equal to the experimental one (q e,exp) at each temperature. The results indicate that Dy(III) adsorption by isoHex-BTP/SiO2-P fits much better to the pseudo-second-order kinetic model than the pseudo-first-order kinetic model. Therefore, it could be concluded that the adsorption is of the chemisorption type due to sharing of electrons between isoHex-BTP and 4f orbital of Dy(III) and the coordination reaction between isoHex-BTP and Dy(III) is the rate-controlling step of the kinetic behavior of adsorption [7, 17, 39]. Furthermore, the goodness of fitting of Dy(III) adsorption is more favorable at higher temperature. q e,exp and q e,calc increased from 0.14 to 0.17 mmol g−1 and from 0.15 to 0.16 mmol g−1, respectively, with increasing the temperature from 288 to 308 K. Presumably the increase in adsorption capacity may be attribute to the strengthening of adsorptive forces between the active sites of isoHex-BTP/SiO2-P and Dy(III) at higher temperatures. Meanwhile, k 2 and h increased dramatically from 1.99 to 26.29 g mmol−1 h–1 and from 0.05 to 0.67 mmol g−1 h−1, respectively, by raising temperature. The increase in adsorption rate is correlated to the acceleration in complexation reaction between Dy(III) and isoHex-BTP/SiO2-P as the rate-controlling step of adsorption and acceleration of some originally slow steps such as diffusion of Dy(III) in solution at higher temperatures.
In view of the adsorption temperature of 288 K, the adsorption kinetic data fit well to both the pseudo-first-order and pseudo-second-order kinetic models with relatively high correlation coefficients (R 2 = 0.983 and 0.988, respectively). One may be tempted to argue that the Dy(III) adsorption by isoHex-BTP/SiO2-P at lower temperatures is more correctly described by more than one adsorption kinetic models.
Adsorption isotherms
Adsorption isotherms describe the relationship between the equilibrium concentration of metal ions in solution and the amount of metal ions adsorbed on a specific adsorbent at a constant temperature. The adsorption isotherms of Dy(III) by isoHex-BTP/SiO2-P as shown in Fig. 7 were obtained from the experimental data in “Effect of initial Dy(III) concentration on adsorption capacity” section. As shown in Fig. 7, the adsorption capacity of Dy(III) increased as the equilibrium concentration of Dy(III) increased, then reached a steady stage which finally reached the maximum adsorption capacity. The equilibrium concentration C e of Dy(III) in solution decreased with increasing temperature, i.e. the adsorption is favored at higher temperatures.
The equilibrium data for Dy(III) adsorption were also analyzed with the most common adsorption isotherm models, the Freundlich and Langmuir isotherm models, to reveal the adsorption mechanism [38, 41].
The Freundlich adsorption isotherm model is used to describe the adsorption of an adsorbate on a heterogeneous surface of an adsorbent. The linear form of the model is given as Eq. (7) [38]:
where K F is a constant related to the maximum adsorption capacity and 1/n F is an empirical parameter related to the adsorption intensity.
Linear plot of logq e versus logC e shows that the adsorption follows the Freundlich isotherm model. K F and n F were calculated from the intercept and the slope of the plot. The parameters n F, K F and R 2 (correlation coefficient) for Dy(III) and isoHex-BTP/SiO2-P system at various temperatures of 288, 298 and 308 K in the Freundlich equation are summarized in Table 3.
The Langmuir adsorption isotherm model is widely used to describe the adsorption of an adsorbate on a homogeneous, flat surface of an adsorbent and each adsorptive site can be occupied only once in a one-on-one manner [38]. Mathematically, the linear form of the model is given in Eq. (8) [39]:
where q e (mmol g−1) is the adsorption capacity at equilibrium, C e (mmol dm–3) is the equilibrium concentration of an adsorbate in solution, q m (mmol g−1) is the maximum adsorption capacity to form a monolayer onto an adsorbent surface, K L (dm3 mmol−1) is Langmuir adsorption constant related to energy of adsorption which quantitatively reflects that affinity between the adsorbent and adsorbate. The fundamental characteristic of a Langmuir isotherm parameter (R L) can be expressed in terms of a dimensionless separation factor or an equilibrium parameter, which is defined by Eq. (9) [38]:
where C 0 (mmol dm−3) is the initial concentration of an adsorbate. According to the value of R L, the shape of the isotherm may be interpreted as follows: R L > 1: unfavorable adsorption, R L = 1: linear adsorption, 0 < R L < 1: favorable adsorption, R L = 0: irreversible adsorption [38].
The linear plot of C e/q e versus C e shows that the adsorption of Dy(III) by isoHex-BTP/SiO2-P obeys Langmuir isotherm model. The values of q m and K L were determined from the slope (1/q m) and intercept (1/K L q m) of the Langmuir plot. Langmuir parameters for fitting adsorption data, q m,calc (the calculated maximum adsorption capacity), K L, R L and R 2 (the correlation coefficient) values at various temperatures are reported in Table 3.
As can be seen, the correlation coefficient (R 2 ≥ 0.999) for the Langmuir isotherm plot was much higher than that for the Freundlich plot (0.621 ≤ R 2 ≤ 0.953) at individual temperature. The calculated maximum adsorption capacity (q m,calc = 0.16, 0.17 and 0.17 mmol g−1 at 288, 298 and 308 K, respectively) from Langmuir isotherm model was equal to the experimental one q m,exp at each individual temperature. The results indicate that the equilibrium adsorption data fit much better to the Langmuir isotherm model than the Freundlich isotherm model, which means that adsorption of Dy(III) occurs on a homogeneous surface by monolayer sorption and each adsorptive site of isoHex-BTP/SiO2-P can be occupied by Dy(III) only once in a one-on-one manner. Both q m,calc and q m,exp slightly increased from 0.16 to 0.17 mmol g−1, with increasing the solution temperature. The increase in maximum adsorption capacity may be due to the higher coordination reaction between Dy(III) and isoHex-BTP/SiO2-P and the higher utilization of all available active sites of isoHex-BTP/SiO2-P for adsorption at higher temperatures. However, the increase in the maximum adsorption capacity with increasing the temperature from 288 to 308 K was very limited (as shown in Table 3). The reasonable conjecture is a saturation adsorption of Dy(III) towards the total existing adsorption sites on internal and external surface area of isoHex-BTP/SiO2-P to form a monolayer adsorption, as discussed in “Effect of initial Dy(III) concentration on adsorption capacity” section. High K L values indicate high adsorption affinity. K L values dramatically increased from 23.44 to 105.64 dm3 mmol−1 with increasing the temperature from 288 to 308 K, suggesting that increasing temperature could increase the affinity of isoHex-BTP/SiO2-P for Dy(III). The values of R L (0 < R L < 1) indicate that the adsorption of Dy(III) by isoHex-BTP/SiO2-P is favorable. Furthermore, R L values decreased with increasing temperature, revealing that the adsorption reaction is more favorable at higher temperature than lower temperature.
Adsorption thermodynamics
From the adsorption kinetics and isotherm results, it can be seen that the increase in temperature generally increased the adsorption properties of Dy(III) by isoHex-BTP/SiO2-P. So values of thermodynamic parameters including enthalpy change (ΔH), entropy change (ΔS) and Gibbs free energy change (ΔG) were determined to confirm the adsorption nature.
In order to obtain the thermodynamic parameters, the temperature dependence of Dy(III) adsorption by isoHex-BTP/SiO2-P at equilibrium was examined at initial Dy(III) concentration of 6 mmol dm–3 in the temperature range from 288 to 308 K. Thermodynamic parameters, ΔH, ΔS and ΔG, associated to the adsorption process were determined using Eqs. (10) and (11) [39, 41]:
where K d, ΔS, ΔH, ΔG, T and R are the distribution coefficient, the entropy change (kJ mol–1 K−1), the enthalpy change (kJ mol−1), Gibbs free energy change (kJ mol−1), absolute temperature (K) and the universal gas constant (0.0083 kJ mol−1 K−1), respectively.
ΔH and ΔS for the adsorption of Dy(III) by isoHex-BTP/SiO2-P can be individually derived from the slope and intercept of the plot of lnK d versus 1/T from Eq. (10). The thermodynamic parameters are summarized in Table 4. Obviously, the negative ΔG value in each individual temperature indicates that the adsorption of Dy(III) by isoHex-BTP/SiO2-P is thermodynamically feasible and spontaneous and the adsorption process would be a product-favored reaction [42]. The ΔG values became more negative with the rise in temperature suggesting that spontaneous nature of adsorption increases at higher temperatures. The positive value of enthalpy change (ΔH = 7.25 kJ mol−1) verifies the endothermic nature of the Dy(III) adsorption by isoHex-BTP/SiO2-P. The positive value of ΔS suggests the increase in the degree of randomness at the solid-solution interface mostly encountered in Dy(III) binding due to the release of water molecules of the hydration sphere [42] during the fixation of Dy(III) on isoHex-BTP/SiO2-P surface [38]. Furthermore, the positive ΔS value, 0.06 kJ mol–1 K–1, indicates that the adsorption process is probably irreversible and favored complexation and stability of adsorption [39]. Although the enthalpy change value was positive, Gibbs free energy change was negative at each individual temperature and the adsorption was spontaneous, which indicates that Dy(III) adsorption by isoHex-BTP/SiO2-P is an entropy-driving process.
Conclusions
Within the scope of this work, the isoHex-BTP/SiO2-P adsorbent exhibited remarkable selectivity and affinity for Am(III) and Pu(IV) over U(VI), Eu(III), Tc(VII) and other typical fission products in 3 mol dm−3 nitric acid solution. Many factors such as contact time, initial concentration of Dy(III) and adsorption temperature had strong impacts on adsorption of Dy(III) (as a simulated element of MA(III) and a representative element of Ln(III)) by isoHex-BTP/SiO2-P from 3 mol dm−3 nitric acid solution. The adsorption data depending on contact time fit much better to the pseudo-second-order kinetic model with high correlation coefficients (0.988 ≤ R 2 ≤ 1.000) than the pseudo-first-order kinetic model, indicating that Dy(III) adsorption by isoHex-BTP/SiO2-P occurs by chemisorption mechanism and the coordination reaction between isoHex-BTP and Dy(III) is the rate-controlling step of the adsorption process. The adsorption data depending on initial Dy(III) concentration were analyzed by the Freundlich and Langmuir isotherm models. The equilibrium adsorption data follow much better the Langmuir isotherm model than the Freundlich isotherm model at the each individual temperature of 288, 298 and 308 K, which means that the adsorption of Dy(III) occurs on a homogeneous surface of isoHex-BTP/SiO2-P and each adsorptive site of isoHex-BTP/SiO2-P can be occupied by Dy(III) only once in a one-on-one manner. The values of Gibbs free energy change (∆G = −10.03, −10.63 and −11.23 kJ mol−1 at 288, 298 and 308 K, respectively), enthalpy change (∆H = 7.25 kJ mol−1) and entropy change (∆S = 0.06 kJ mol−1 K−1) indicate that the adsorption process is spontaneous, the endothermic nature and an entropy-driving process. These results also revealed that increasing temperature is benefit to the adsorption process of Dy(III) towards isoHex-BTP/SiO2-P by increasing the equilibrium adsorption capacity, the maximum adsorption capacity, the adsorption rate, the adsorption affinity and the spontaneous nature of adsorption process.
The results demonstrated that isoHex-BTP/SiO2-P is a promising adsorbent for the direct separation of Am(III) and residual Pu(IV) from Ln(III) and other typical elements present in HLLW. Through this study, essential adsorption kinetics, adsorption isotherm and thermodynamics data were collected for an engineering design of the column separation of MA(III) from HLLW by isoHex-BTP/SiO2-P. In addition, more detailed property evaluations of isoHex-BTP/SiO2-P by column separation experiments are under the way.
References
Madic C, Boullis B, Baron P, Testard R, Hudson MJ, Lijenzin JO, Christiansen B, Ferrando M, Facchini A, Geist A, Modolo G, Espartero AG, De Mendoza J (2007) Futuristic back-end of the nuclear fuel cycle with the partitioning of minor actinides. J Alloys Compd 444:23–27
Madic C, Hudson MJ, Liljenzin JO, Glatz JP, Nannicini R, Facchini A, Kolarik Z, Odoj R (2002) Recent achievements in the development of partitioning processes of minor actinides from nuclear wastes obtained in the frame of the NEWPART European programme (1996–1999). Prog Nucl Energy 40:523–526
Malmbeck R, Courson O, Pagliosa G, Romer K, Satmark B, Glatz JP, Baron P (2000) Partitioning of minor actinides from HLLW using the DIAMEX process. Part 2—“Hot” continuous counter-current experiment. Radiochim Acta 88:865–871
Salvatores M, Palmiotti G (2011) Radioactive waste partitioning and transmutation within advanced fuel cycles: achievements and challenges. Prog Part Nucl Phys 66:144–166
Trumm S, Geist A, Panak PJ, Fanghanel T (2011) An improved hydrolytically-stable Bis-Triazinyl-Pyridine (BTP) for selective actinide extraction. Solvent Extr Ion Exch 29:213–229
Geist A, Hill C, Modolo G, Foreman M, Weigl M, Gompper K, Hudson MJ (2006) 6,6′-bis (5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-benzo [1, 2, 4] triazin-3-yl) [2,2′] bipyridine, an effective extracting agent for the separation of americium(III) and curium(III) from the lanthanides. Solvent Extr Ion Exch 24:463–483
Denecke MA, Rossberg A, Panak PJ, Weigl M, Schimmelpfennig B, Geist A (2005) Characterization and comparison of Cm(III) and Eu(III) complexed with 2,6-di(5,6-dipropyl-1,2,4-triazin-3-yl)pyridine using EXAFS, TRFLS, and quantum-chemical methods. Inorg Chem 44:8418–8425
Mathur JN, Murali MS, Ruikar PB, Nagar MS, Sipahimalani AT, Bauri AK, Banerji A (1998) Degradation, cleanup, and reusability of octylphenyl-N, N′-diisobutylcarbamoylmethyl phosphine oxide (CMPO) during partitioning of minor actinides from high level waste (HLW) solutions. Sep Sci Technol 33:2179–2196
Patil AB, Shinde VS, Pathak PN, Mohapatra PK, Manchanda VK (2013) Modified synthesis scheme for N, N′-dimethyl-N, N′-dioctyl-2, (2′-chexyloxyethyl) malonamide (DMDOHEMA) and its comparison with proposed solvents for actinide partitioning. Radiochim Acta 101:93–100
Chen J, Wang JC (2011) Overview of 30 years research on TRPO process for actinides partitioning from high level liquid waste. Prog Chem 23:1366–1371
Paiva AP, Malik P (2004) Recent advances on the chemistry of solvent extraction applied to the reprocessing of spent nuclear fuels and radioactive wastes. J Radioanal Nucl Chem 261:485–496
Ansari SA, Pathak P, Mohapatra PK, Manchanda VK (2011) Aqueous partitioning of minor actinides by different processes. Sep Purif Rev 40:43–76
Whittaker DM, Griffiths TL, Helliwell M, Swinburne AN, Natrajan LS, Lewis FW, Harwood LM, Parry SA, Sharrad CA (2013) Lanthanide speciation in potential SANEX and GANEX actinide/lanthanide separations using tetra-N-donor extractants. Inorg Chem 52:3429–3444
Modolo G, Wilden A, Daniels H, Geist A, Magnusson D, Malmbeck R (2013) Development and demonstration of a new SANEX partitioning process for selective actinide(III)/lanthanide(III) separation using a mixture of CyMe4BTBP and TODGA. Radiochim Acta 101:155–162
Bhattacharyya A, Mohapatra PK, Manchanda VK (2007) Solvent extraction and extraction chromatographic separation of Am3+ and Eu3+ from nitrate medium using Cyanex® 301. Solvent Extr Ion Exch 25:27–39
Bhattacharyya A, Mohapatra PK, Ansari SA, Raut DR, Manchanda VK (2008) Separation of trivalent actinides from lanthanides using hollow fiber supported liquid membrane containing Cyanex-301 as the carrier. J Membr Sci 312:1–5
Panak PJ, Geist A (2013) Complexation and extraction of trivalent actinides and lanthanides by triazinylpyridine N-Donor ligands. Chem Rev 113:1199–1236
Weigl M, Geist A, Mullich U, Gompper K (2006) Kinetics of americium(III) extraction and back extraction with BTP. Solvent Extr Ion Exch 24:845–860
Kaltsoyannis N (2013) Does covalency increase or decrease across the actinide series? Implications for minor actinide partitioning. Inorg Chem 52:3407–3413
Guillaumont D (2006) Actinide(III) and lanthanide(III) complexes with nitrogen ligands: counterions and ligand substituent effects on the metal-ligand bond. Theochem J Mol Struct 771:105–110
Nilsson M, Andersson S, Ekberg C, Foreman MRS, Hudson MJ, Skarnemark G (2006) Inhibiting radiolysis of BTP molecules by addition of nitrobenzene. Radiochim Acta 94:103–106
Takeshita K, Matsumura T, Nakano Y (2008) Separation of americium(III) and europium(III) by thermal-swing extraction using thermosensitive polymer gel. Prog Nucl Energy 50:466–469
Bhattacharyya A, Mohapatra PK, Roy A, Gadly T, Ghosh SK, Manchanda VK (2009) Ethyl-bis-triazinylpyridine (Et-BTP) for the separation of americium(III) from trivalent lanthanides using solvent extraction and supported liquid membrane methods. Hydrometallurgy 99:18–24
Matsumura T, Matsumura K, Morita Y, Koma Y, Sano Y, Nomura K (2011) Separation of trivalent minor actinides from fission products using single R-BTP column extraction chromatography. J Nucl Sci Technol 48:855–858
Van Hecke K, Modolo G (2004) Separation of actinides from low level liquid wastes (LLLW) by extraction chromatography using novel DMDOHEMA and TODGA impregnated resins. J Radioanal Nucl Chem 261:269–275
Bhattacharyya A, Mohapatra PK, Gadly T, Ghosh SK, Raut DR, Manchanda VK (2011) Extraction chromatographic study on the separation of Am3+ and Eu3+ using ethyl-BTP as the extractant. J Radioanal Nucl Chem 288:571–577
Mohapatra P, Sriram S, Manchanda V, Badheka L (2000) Uptake of metal ions by extraction chromatography using dimethyl dibutyl tetradecyl-1,3-malonamide (DMDBTDMA) as the stationary phase. Sep Sci Technol 35:39–55
Liu RQ, Wei YZ, Tozawa D, Xu YL, Usuda S, Yamazaki H, Ishii K, Sano Y, Koma Y (2011) Evaluation study on properties of a macroporous silica-based CMPO extraction resin to be used for minor actinides separation from high level liquid waste. Nucl Sci Technol 22:18–24
Liu RQ, Wei YZ, Xu YL, Usuda S, Kim S, Yamazaki H, Ishii K (2012) Evaluation study on properties of isohexyl-BTP/SiO2-P resin for direct separation of trivalent minor actinides from HLLW. J Radioanal Nucl Chem 292:537–544
Usuda S, Wei Y, Xu Y, Li Z, Liu R, Kim S, Wakui Y, Hayashi H, Yamazaki H (2012) Development of a simplified separation process of trivalent minor actinides from fission products using novel R-BTP/SiO2-P adsorbents. J Nucl Sci Technol 49:334–342
Wei YZ, Sabharwal KN, Kumagai M, Asakura T, Uchiyama G, Fujine S (2000) Preparation of novel silica-based nitrogen donor extraction resins and their adsorption performance for trivalent americium and lanthanides. J Nucl Sci Technol 37:1108–1110
Wei Y, Kumagai M, Takashima Y, Modolo G, Odoj R (2000) Studies on the separation of minor actinides from high-level wastes by extraction chromatography using novel silica-based extraction resins. Nucl Technol 132:413–423
Wei YZ, Hoshi H, Kumagai M, Asakura T, Morita Y (2004) Separation of Am(III) and Cm(III) from trivalent lanthanides by 2,6-bistriazinylpyridine extraction chromatography for radioactive waste management. J Alloys Compd 374:447–450
Liu RQ, Wang XP, Wei YZ, Shi WQ, Chai ZF (2014) Evaluation study on a macroporous silica-based isohexyl-BTP adsorbent for minor actinides separation from nitric acid medium. Radiochim Acta 102:93–100
Gorden AEV, DeVore MA, Maynard BA (2013) Coordination chemistry with f-element complexes for an improved understanding of factors that contribute to extraction selectivity. Inorg Chem 52:3445–3458
Bozic D, Stankovic V, Gorgievski M, Bogdanovic G, Kovacevic R (2009) Adsorption of heavy metal ions by sawdust of deciduous trees. J Hazard Mater 171:684–692
Semerjian L (2010) Equilibrium and kinetics of cadmium adsorption from aqueous solutions using untreated Pinus halepensis sawdust. J Hazard Mater 173:236–242
Javadian H, Ghaemy M, Taghavi M (2014) Adsorption kinetics, isotherm, and thermodynamics of Hg2+ to polyaniline/hexagonal mesoporous silica nanocomposite in water/wastewater. J Mater Sci 49:232–242
Dikici H, Saltali K, Bingolbali S (2010) Equilibrium and kinetics characteristics of copper (II) sorption onto gyttja. Bull Environ Contam Toxicol 84:147–151
Hanafiah M, Zakaria H, Ngah WSW (2009) Preparation, characterization, and adsorption behavior of Cu(II) ions onto alkali-treated weed (imperata cylindrica) leaf powder. Water Air Soil Pollut 201:43–53
Ozeroglu C, Metin N (2012) Adsorption of uranium ions by crosslinked polyester resin functionalized with acrylic acid from aqueous solutions. J Radioanal Nucl Chem 292:923–935
Salam MA (2013) Removal of heavy metal ions from aqueous solutions with multi-walled carbon nanotubes: kinetic and thermodynamic studies. Int J Environ Sci Technol 10:677–688
Acknowledgments
This work was supported by National Natural Science Foundation of China (Grant No. 11305102, 91126006, 21261140335, 91226111) and Doctoral Fund of Ministry of Education of China (Grant No. 20130073110046).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, R., Ning, S., Wang, X. et al. Adsorption behavior of actinides and some typical fission products by silica/polymer-based isoHex-BTP adsorbent from nitric acid solution. J Radioanal Nucl Chem 303, 681–691 (2015). https://doi.org/10.1007/s10967-014-3472-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3472-8