Abstract
A silica/polymer-based CA-BTP/SiO2–P adsorbent was prepared to separate minor actinides and some key radionuclides from HLLW. The adsorption properties of CA-BTP/SiO2–P toward 238U(VI), 239Pu(IV), 241Am(III), 99Tc(VII), 152Eu(III), and some typical fission products were studied. CA-BTP/SiO2–P stability against γ-radiation was also evaluated. It found CA-BTP/SiO2–P showed very poor adsorption abilities toward U(VI) and most experimental FPs, while CA-BTP/SiO2–P exhibited higher adsorption abilities toward 241Am(III), 239Pu(IV), and 99Tc(VII) in 0.5–1 M HNO3 solution. Moreover, dry CA-BTP/SiO2–P demonstrated no instability when the radiation dose was up to 161 kGy. CA-BTP/SiO2–P adsorbent is a potential candidate for separating 241Am(III), 239Pu(IV), and 99Tc(VII) from HLLW.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Minor actinides (MA: Np, Am, Cm, etc.), small amount of residual plutonium, and long-lived fission products (FPs) are the main contributors of long-term radioactivity of high level liquid wastes (HLLW) produced from the PUREX spent fuel reprocessing process. Partitioning MA, Pu, and long-lived FPs from HLLW and transmuting them into short-lived or stable nuclides in a fast reactor or accelerator driven system (ADS) is a key step in reducing the long-term radiotoxicity of HLLW. Partitioning and transmutation could facilitate the long-term management of HLLW and improve the public acceptance of civilian nuclear energy [1, 2]. To enhance the transmutation efficiency of MA, it is necessary to separate MA from lanthanides (Ln), since several Ln are neutron absorbers with large capture cross-sections [3].
However, MA(III) has the same trivalent state as well as comparable and overlapping ionic radii as Ln(III), which leads to their similar chemical properties and makes the separation from Ln(III) difficult. Currently, two-step-separation processes are considered. First, Ln and MA are co-separated from high acidity HLLW by means of the traditional O-donor extracting agents, e.g., CMPO [4], TODGA [5], TRPO [6], and DIDPA [7] in the SETFICS, TRUEX, TRPO, and DIDPA processes, etc. These adsorbents show good stabilities against nitric acid and radiation, but poor selectivity between MA and Ln. Second, MA could be selectively separated from Ln using the N- or S- donor ligands in CYANEX 301 [8] and SANEX [9] processes, etc.
A new kind of N-donor ligands, BTPs (BTP: 2,6-bis-(5,6-dialkyl-1,2,4-triazin-3-yl)-pyridines), first used for MA(III) separation from HLLW by Kolarik [10, 11], has been studied widely due to its high extraction selectivity for Am(III) over Eu(III). The separation factor SF Am/Eu has been obtained around 100 in a wide range of nitric acid concentration ([HNO3]: 0.01–4 M, M = mol L−1). Moreover, the ligands contain only C, H, and N elements and fulfill the CHON principle, allowing them to be completely combustible into gaseous products after use. However, BTPs with simple n-alkyl ligand groups, e.g., n-propyl-BTP and n-butyl-BTP, have low stability against nitric acid and/or radiation. The instability is caused by the abstraction of hydrogen from the pseudo-benzylic position of the triazine rings, followed by the forming of alcohols and ketones and ultimately leading to the loss of alkyl chains and decay of the extractants [9, 12]. To overcome the drawback of instability, a series of modified BTPs has been synthesized, such as isopropyl-BTP [9], isobutyl-BTP [13], and isohexyl-BTP [14–17] with branched alkyl structures or CyMe4-BTP [9, 12] and Me2-CA-BTP [18] with cycle structures, replacing the methyl groups and avoiding the pseudo-benzylic hydrogen. They all show improvement on performance in chemical and/or radiation stability.
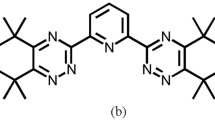
CA-BTP (bis-2,6-(5,6,7,8-tetrahydro-5,9,9-trimethyl-5,8-methano-1,2,4-benzotriazin-3-yl)pyridine) with side cycle structures shown in Fig. 1a is one of the most potential candidates for MA and Ln separation from HLLW [12]. The extraction properties of CA-BTP toward 241Am(III) and 152Eu(III) using liquid–liquid extraction technology were studied by S. Trumm et al. [19]. The study found that CA-BTP had good acid resistance, high solubility in 1-octanol, good selectivity, and satisfactory extraction kinetic. Considering that MA content in HLLW is much less than those of U and Pu in the spent fuel, the scale of the separation of MA from HLLW would be considerably smaller than that of PUREX. Wei et al. proposed a separation process for MA and other key radionuclides from HLLW based on the extraction chromatography technology [20]. The extraction chromatography technology consists of two main parts. First, obtaining the basic adsorption and separation information, such as the optimum nitric acid concentration for adsorption and separation, the adsorption kinetic and desorption kinetic, adsorption isotherm, thermodynamics, and stability by means of batch adsorption experiments. Second, the continuous chromatography separation process which includes three principle steps, i.e., pre-equilibration step using the same media as the following adsorption step, but without the target metal elements in the solution, followed by the adsorption step using the media in which some elements will be adsorbed onto the adsorbent in the column and the non-adsorbed elements will just flow through as the solution, and the desorption step in which the adsorbed elements will be washed down from the column step by step using different eluents based on their different affinities between the adsorbates and adsorbent. Compared with the liquid–liquid extraction method, the extraction chromatography process uses almost no organic solution in the separation process and hence avoids a large amount of secondary organic waste accumulation. Due to the complexity of HLLW, more than one chromatography column may be required for the separation.
In this work, a new kind of CA-BTP/SiO2–P adsorbent was synthesized by impregnating CA-BTP molecules into macro-porous and stable silica/polymer-based support particles (SiO2–P) for the separation of MA and some key radionuclides from simulated HLLW by extraction chromatography batch experiments. The adsorption properties of CA-BTP/SiO2–P toward 238U(VI), 239Pu(IV), 241Am(III), 99Tc(VII), 152Eu(III), and some typical FPs in a wide range of HNO3 concentration were evaluated. The adsorption behavior of Y(III), Ln(III) in 1 M HNO3 solution and the adsorption kinetic of 241Am(III), 152Eu(III) were also evaluated. In addition, the stability of the dry CA-BTP/SiO2–P adsorbent against γ-radiation was investigated.
Experimental
Reagents
The chemical reagents Ln(NO3)3·nH2O (where Ln = Y, La, Ce, Pr, Nd, Sm, Eu, Gd, Dy, Er, Yb, and n = 5–6), Ho (Ho2O3), Tm (Tm2O3), and FP nitrates (where FP = Sr(II), Zr(IV), Cs(I)) were of analytical grade. Ru(III) nitrosyl nitrate solution was in diluted nitric acid containing 1.5 wt% of Ru(III) with the density of 1.07 g mL−1. The radionuclides 238U(VI), 239Pu(IV), 241Am(III), 99Tc(VII), and 152Eu(III) were obtained from China Institute of Atomic Energy (CIAE). The purity of CA-BTP was 95 %. Other agents such as nitric acid and dichloromethane were of analytical grade and used without further treatment.
Synthesis and characterization of CA-BTP/SiO2–P adsorbent
The silica/polymer-based composite support particles (SiO2–P) with the pore size of 0.6 μm, pore fraction of 0.69, and mean diameter of 60 μm have been developed in previous works [20]. P refers to macroreticular styrene–divinylbenzene copolymer (SDB) and is immobilized in porous silica (SiO2) particles. The synthesis procedure of CA-BTP/SiO2–P adsorbent was similar with that in [21]. First, the macro-porous SiO2–P particles were washed with methanol three times and dried in vacuum at 40 °C overnight. Second, 5 g of CA-BTP extractant was weighed, placed in a flask, and dissolved in dichloromethane. Subsequently, 10 g of SiO2–P particles was added to the solution and the mixture was shaken vigorously for 1.5 h at room temperature. Then, the mixture was still for 1 h. Afterwards, the diluent was removed at 40 °C under reduced pressure by a rotary evaporator to impregnate and immobilize CA-BTP into the pores of SiO2–P particles. Then, the mixture was dried in vacuum at 40 °C overnight. Finally, the silica-based CA-BTP/SiO2–P adsorbent was obtained.
The synthetic CA-BTP/SiO2–P adsorbent was characterized by the high-resolution field emission scanning electron microscope (SEM, FEI COMPANY Sirion 200) and the SEM image is shown in Fig. 1b. Both SiO2–P particles and CA-BTP/SiO2–P adsorbent composition were analyzed by the thermal gravimetry and differential thermal analysis (TG–DTA, Shimadzu DTG-60) and the results are shown in Fig. 2. The TG–DTA curves of SiO2–P shown in Fig. 2a indicate that the content of P in SiO2–P was around 16.9 wt% and the thermal decomposition began at about 260 °C, followed by an exothermic peak, and sustained to about 530 °C. The TGA curves of CA-BTP/SiO2–P adsorbent are presented in Fig. 2b, in which the thermal decomposition of the CA-BTBP/SiO2–P adsorbent began at about 260 °C and the decomposition process sustained to 530 °C. The CA-BTP/SiO2–P adsorbent lost 43.3 wt% of its original weight when it was heated up to 570 °C. According to results above, the mass composition of CA-BTP/SiO2–P was determined to contain 31.8, 11.5, and 56.7 wt% of CA-BTP compound, SDB polymer, and SiO2, respectively.
Batch adsorption experiments
The adsorption properties of CA-BTP/SiO2–P adsorbent toward 238U(VI), 239Pu(IV), 241Am(III), 99Tc(VII), 152Eu(III), and some typical FPs were evaluated by batch adsorption experiments. A definite amount of adsorbent (e.g., 0.1 g) was mixed in a volume (e.g., 5 mL) of aqueous solution in a glass vial with a screw cap. The vial was shaken mechanically at 120 rpm in a water bath at 25 °C for a pre-determined time. Then, the aqueous phase was filtrated through a membrane filter with 0.25 μm mesh. The radioactivities of 241Am(III) and 152Eu(III) in the solution before and after adsorption were determined by the high-purity germanium multichannel gamma spectrometer (GEM70P-PLUS, ORTEC) and the radioactivities of 99Tc(VII) and 239Pu(IV) were measured by Super Low Level Liquid Scintillation Analyzer (PE Tri-Carb 3170). The concentration of 238U(VI) was measured by the UV spectrophotometer (Lab Tech UV1000/1100). The Cs(I) concentration was determined by the atomic absorption spectrophotometer (AAS, SP-3801). The concentrations of the other FP elements were analyzed by the inductively coupled plasma atomic emission spectroscopy (ICP-AES: Shimadzu ICPS-7510). The distribution coefficient K d (mL g−1), separation factor SF A/B, and uptake rate were calculated by Eqs. (1), (2), and (3), respectively:
where C o or A o, and C e or A e (in mM or Bq·mL−1) denote the concentration or activity of metal in the aqueous phase before and after adsorption, respectively. V (mL) indicates the volume of aqueous phase and W (g) is the mass of dry CA-BTP/SiO2–P adsorbent.
Stability of CA-BTP/SiO–P against γ-radiation
To investigate the stability of CA-BTP/SiO2–P adsorbent against γ radiation, dry CA-BTP/SiO2–P adsorbent was packed in glass vials and irradiated by 60Co-γ source with the radiation dose rate of 1 kGy h−1. After receiving the predetermined radiation doses, the CA-BTP/SiO2–P adsorbent was analyzed by the ultraviolet and visible spectrophotometer (Shimadzu UV-3600) to check the changes in structure and was used for the adsorption of 241Am(III) and 152Eu(III) in batch experiments to exam the property changes in adsorption and selectvitity.
Results and discussion
Effect of nitric acid concentration on adsorption and selectivity
The effect of initial nitric acid concentration on the adsorption of CA-BTP/SiO2–P toward actinides, e.g., 238U(VI), 239Pu(IV), 241Am(III), and typical FP, e.g., Sr(II), Cs(I), Zr(IV), Ru(III), Y(III), Ln(III), as well as some trace amount of radionuclides 152Eu(III) and 99Tc(VII) was evaluated in 0.01–5 M HNO3 solution. The results are shown in Fig. 3. This figure indicates that, within the experimental range of HNO3 concentration, CA-BTP/SiO2–P adsorbent showed poor or almost no adsorption abilities toward Sr(II), Cs(I), Y(III), the experimental Ln(III), 238U(VI), and 152Eu(III) with K d values less than 5, where the results of Sr(II), Cs(I), Y(III), 238U(VI), and the lighter Ln(III) were superimposed by Dy(III). For elements 239Pu(IV), 241Am(III), 99Tc(VII), and Zr(IV), the adsorption behavior followed a similar pattern, in which K d values first increased with increasing nitric acid concentration up to 0.5–1.0 M and then decreased with the further increase of nitric acid concentration. The reason for this behavior may be that CA-BTP/SiO2–P adsorption toward the above-mentioned few metal ions requires H+ or NO3 − participation, so, the adsorption increased with increasing nitric acid concentration. However, as the co-extraction of HNO3 by BTP reduced the free BTP available at elevated nitric acid concentration, the adsorption decreased as the nitric acid concentration further increased [22]. Also, considering the complicated hydrolysis states of the metal ions in solution when the nitric acid concentration changed, e.g., M(OH) 4−i i (where M = 239Pu(IV), Zr(IV), and i = 0, 1, 2, 3, 4, where i decreases as nitric acid increases and the ionic radius of M(OH) 4−i i increases as i increases) [23, 24], only one or two hydrolysis states might be favored by CA-BTP/SiO2–P adsorbent. Similar results were found in liquid–liquid extraction in [19] where the extraction of CA-BTP toward 241Am(III) first increased with the increase of the nitric acid concentration and then reached the maximum at about 1 M HNO3 and decreased as the nitric acid concentration further increased. The results in [19] also showed that extraction toward 152Eu(III) was very poor. Also, CA-BTP/SiO2–P was a potential adsorbent for separating 239Pu(IV), 241Am(III), and 99Tc(VII) from most FPs from HLLW within the nitric acid range of 0.5–1 M and the following desorption can be realized by using water or dilute nitric acid as eluent.
Adsorption in single target element system in 1 M HNO3
In the second step of the two-step-separation processes for separating MA(III) from HLLW, MA(III) is separated from Y(III) and other fission-product Ln(III) in a low nitric acid solution. According to Fig. 3, CA-BTP/SiO2–P adsorbent had relatively good adsorption abilities toward 239Pu(IV) and 241Am(III) in 0.5–1.0 M HNO3 solution. The adsorption behavior of CA-BTP/SiO2–P toward Y(III) and almost all Ln(III) in a single target element system was studied individually in 1.0 M HNO3. The K d values of Y(III), 239Pu(IV), 241Am(III), and almost all Ln(III) were compared and shown in Fig. 4. It indicates that CA-BTP/SiO2–P had almost no adsorption toward Ln(III), lighter than Dy(III). For Dy(III) and other heavier Ln(III), CA-BTP/SiO2–P showed some adsorption and the adsorption abilities increased as Ln(III) atomic number increased. For 239Pu(IV) and 241Am(III), the adsorption was better than those of Y(III) and all the experimental Ln(III). The results were consistent with those shown in Fig. 3. Since HLLW contains little or almost no Dy(III) and Ln heavier than Dy, their effects on the separation of MA from HLLW using CA-BTP/SiO2–P would be slight. Therefore, it is feasible for CA-BTP/SiO2–P to be used as an adsorbent for separating 241Am(III), 99Tc(VII), and the residual 239Pu(IV) from fission products from HLLW, especilly for separating 241Am(III) from Y(III) and Ln(III) fission products from HLLW in the second step of the two-step-separation processes mentioned above.
Effect of contact time on adsorption toward 241Am(III) and 152Eu(III)
The adsorption kinetic of CA-BTP/SiO2–P toward 241Am(III) and 152Eu(III) in 0.5 M HNO3 was studied. The distribution coefficients and uptake rate of 241Am(III) and 152Eu(III) as a function of contact time are presented in Fig. 5. It indicates that CA-BTP/SiO2–P showed almost no adsorption toward 152Eu(III) as a function of contact time within the 24 h experimental time. For 241Am(III), the distribution coefficient (K d) and the uptake rate increased slowly from 0.5 to 24 h, but still did not reach equilibrium within the 24 h experimental time.
Although CA-BTP/SiO2–P adsorption toward 241Am(III) did not reach equilibrium within the 24 h experimental time, CA-BTP/SiO2–P is a potential candidate for separating 241Am(III) from HLLW. Figure 5 shows that the adsorption kinetic toward 241Am(III) was relatively fast during the initial 0.5 h contact time with the uptake rate reaching 43 %. The reasons may be that, at the very beginning of the adsorption, a large number of vacant surface sites were available for the adsorption of 241Am(III). As more 241Am(III) was adsorbed, the remaining vacant surface sites were more difficult to be occupied due to the repulsive forces between the solute molecules on the solid surface and the bulk phase. Also, 241Am(III) was adsorbed and saturated in the meso-pores and encountered more resistance as it traversed deeper into the pores of the adsorbent [25]. In addition, the concentration of 241Am(III) in the solution decreased as a function of contact time; hence, the available 241Am(III) for adsorption also decreased.
Stability of CA-BTP/SiO2–P against γ-radiation
Besides selectivity, the adsorbent stability against nitric acid and radiation is also important for practical applications. The stability of CA-BTP against nitric acid was studied [19] and good stability over a long time period against nitric acid was achieved. Herein, the stability of CA-BTP/SiO2–P adsorbent against γ-radiation was focused on. Dry CA-BTP/SiO2–P adsorbent was packed in glass vials and irradiated by 60Co-γ source with the radiation dose rate of 1 kGy h−1. After being irradiated to the predetermined doses, CA-BTP/SiO2–P adsorbent was analyzed using ultraviolet (UV) and visible spectrophotometer and was used in batch experiments for adsorption toward 241Am(III) and 152Eu(III).
The samples for UV test were prepared by dissolving 10 mg CA-BTP/SiO2–P adsorbent into 10 mL acetonitrile where the CA-BTP molecules were dissolved into acetonitrile. The supernatant liquor was further diluted for 25 times with acetonitrile, i.e., the CA-BTP molecule concentration for the final UV test was about 2.9 × 10−5 mol mL−1. The results of UV test are shown in Fig. 6. This figure indicates that the CA-BTP molecules showed no damage sign by γ-irradiation in the experiment as the curves with different radiation doses and the curve without irradiation were almost completely overlapped within the experiment error. Furthermore, batch adsorption experiments using the γ-irradiated adsorbent for adsorption toward 241Am(III) and 152Eu(III) were conducted in 0.5 M HNO3 with the contact time of 24 h. Results are shown in Fig. 7, representing that the uptake rates of 152Eu(III) and 241Am(III) showed almost no changes as the radiation dose increased.
Conclusions
The CA-BTP/SiO2–P adsorbent was synthesized for the purpose of separating minor actinides and some key long-lived radionuclides from HLLW using the extraction chromatography method. The adsorption behavior of CA-BTP/SiO2–P toward 238U(VI), 239Pu(IV), 241Am(III), 99Tc(VII), 152Eu(III), and some typical FPs was studied in batch adsorption experiments. It was found that CA-BTP/SiO2–P showed almost no adsorption toward most FPs, while its adsorption abilities toward 239Pu(IV), 241Am(III), and 99Tc(VII) were relatively good in 0.5–1 M HNO3 solution. The results indicated that CA-BTP/SiO2–P can be a potential adsorbent for the separation of 239Pu(IV), 241Am(III), and 99Tc(VII) from HLLW, especially for the use in the second step separation process of the two-step-separation processes for separating MA(III) from Y(III) and Ln(III) from HLLW. Stability against γ-radiation was also evaluated and the results indicated that dry CA-BTP/SiO2–P adsorbent showed good stability against γ-radiation when the radiation dose was up to 161 kGy.
Based on the above results, the future work will mainly focus on the continuous chromatography separation experiment for separating MA(III) from Y(III) and Ln(III) from HLLW in the second step process of the two-step-separation processes mentioned above. Furthermore, 239Pu(IV) and 99Tc(VII) can also be removed together if necessary. The appropriate media for the pre-equilibration and adsorption steps is 0.5–1 M HNO3, and water will be a good candidate of eluent for the desorption of the adsorbed 241Am(III), 239Pu(IV), and 99Tc(VII).
References
Magill J, Berthou V, Haas D, Galy J, Schenkel R, Wiese HW, Heusener G, Thommasi J, Youinou G (2003) Impact limits of partitioning and transmutation scenarios on the radiotoxicity of actinides in radioactive waste. Nucl Energy 42:263–277
OECD-NEA (1999) Status and assessment report on actinide fission product partitioning and transmutation
Malmbeck R, Nourry C, Ougier M, Souček P, Glatz JP, Kato T, Koyama T (2011) Advanced fuel cycle options. Energy Procedia 7:93–102
Liu RQ, Wei YZ, Daisuke T, Xu YL, Usuda S, Hiromichi Y, Keizo I, Sano Y, Yoshikazu K (2011) Evaluation study on properties of a macroporous silica based CMPO. Nucl Sci Technol 22:18–24
Xu YL, Wei YZ, Liu RQ, Usuda S, Ishii K, Yamazaki H (2011) Adsorption characteristics of trivalent rare earths and chemical stability of a silica-based macroporous TODGA adsorbent in HNO3 solution. J Nucl Sci Technol 48(9):1223–1229
Liu XG, Liang JF, Xu JM (2004) Simplified chinese TRPO process to extract and recover transuranium elements from high level liquid waste. Solvent Extr Ion Exch 22(2):163–173
Paiva AP, Malik P (2004) Recent advances on the chemistry of solvent extraction applied to the reprocessing of spent nuclear fuels and radioactive wastes. J Radioanal Nucl Chem 261:485–496
Zhu Y, Chen J, Jiao R (1996) Extraction of Am(III) and Eu(III) from nitrate solution with purified cyanex 301. Solvent Extr Ion Exch 14:61–68
Clément H, Guillaneux D, Berthon L, Madic C (2002) SANEX-BTP process development studies. J Nucl Sci Technol 39(Supplement 3):309–312
Kolarik Z, Udo M, Franz G (1999) Selective extraction of Am(III) over Eu(III) by 2,6-ditriazolyl- and 2,6-ditriazinylpyridines. Solvent Extr Ion Exch 17:23–32
Kolarik Z, Udo M, Franz G (1999) Extraction of Am(III) and Eu(III) nitrates by 2-6-Di-(5,6-dipropyl-1,2,4-triazin-3-Yl)pyridines. Solvent Extr Ion Exch 17:1155–1170
Panak PJ, Geist A (2013) Complexation and extraction of trivalent actinides and lanthanides by triazinylpyridine N-donor ligands. Chem Rev 113:1199–1236
Xu YL, Kim SY, Ito T, Hitomi K, Kuraoka E, Usuda S, Ishii K (2013) Adsorption behavior of trivalent americium and rare earth ions onto a macroporous silica-based isobutyl-BTP/SiO2–P adsorbent in nitric acid solution. J Radioanal Nucl Chem 299(1):149–155
Wang XP, Ning SY, Liu RQ, Wei YZ (2014) Stability of isohex-BTP SiO2–P adsorbent against acidic hydrolysis and Γ-irradiation. Sci China Chem 57(11):1464–1469
Liu RQ, Ning SY, Wang XP, Wei YZ, Yang JL, Zhao YP, Ding YQ, Lan JH, Shi WQ (2014) Adsorption behavior of actinides and some typical fission products by silica/polymer-based isohex-BTP adsorbent from nitric acid solution. J Radioanal Nucl Chem 303(1):681–691
Wei YZ, Wang XP, Liu RQ, Wu Y, Shigekazu U, Tsuyoshi A (2012) An advanced partitioning process for key elements. Sci China Chem. 55(9):1726–1731
Liu RQ, Wei YZ, Xu YL, Shigekazu U, Seongyun K, Hiromichi Y, Keizo I (2012) Evaluation study on properties of isohexyl-BTP/SiO2–P resin for direct separation of trivalent minor actinides from HLLW. J Radioanal Nucl Chem 292:537–544
Ning SY, Wang XP, Liu RQ, Wei YZ, He LF, Tang FD (2014) Evaluation of Me2-CA-BTP/SiO2–P adsorbent for the separation of minor actinides from simulated HLLW. J Radioanal Nucl Chem 303(3):2011–2017
Trumm S, Geist A, Panak PJ, Fanghänel T (2011) An improved hydrolytically-stable bis-triazinyl-pyridine (BTP) for selective actinide extraction. Solvent Extr Ion Exch 29:213–229
Wei YZ, Kanwal NS, Kumagai M, Asakura T, Uchiyama G, Fujine S (2000) Preparation of novel silica-based nitrogen donor extraction resins and their adsorption performance for trivalent americium and lanthanide. J Nucl Sci Technol 37:1108–1110
Wei YZ, Hoshi H, Kumagai M, Asakura T, Morita Y (2004) Separation of Am(III) and Cm(III) from trivalent lanthanides by 2,6-bistriazinylpyridine extraction chromatography for radioactive waste management. J Alloy Compd 374:447–450
Weigl M, Geist A, Müllich U, Gompper K (2006) Kinetics of americium(III) extraction and back extraction with BTP. Solvent Extr Ion Exch 24:845–860
Solovkin AS (1974) Thermodynamics of extraction of tetravalent plutonium, uranium, thorium and zirconium. J Radioanal Nucl Chem 21:15–29
Fujii T, Hajimu Y, Masayuki W, Hirotake M (2002) Extraction study for truex process using short-lived radionuclides produced by neutron irradiation of uranium. Solvent Extr Ion Exch 20(2):151–175
Srivastava VC, Mall ID, Mishra IM (2006) Characterization of mesoporous rice husk ash (Rha) and adsorption kinetics of metal ions from aqueous solution onto rha. J Hazard Mater 134(1–3):257–267
Acknowledgments
This study was carried out under the support of National Natural Science Foundation with the Project No. 91126006, No.11305102, and the support of the Ministry of Education of Specialized Research Fund for the Doctoral Program of Higher Education with the Project No. 20130073110046.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ning, S., Zou, Q., Wang, X. et al. Evaluation study on silica/polymer-based CA-BTP adsorbent for the separation of minor actinides from simulated high-level liquid wastes. J Radioanal Nucl Chem 307, 993–999 (2016). https://doi.org/10.1007/s10967-015-4248-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-015-4248-5