Abstract
Non-invasive quantification of myocardial β-receptors could become an independent prognostic marker for chronic heart failure and cardiovascular disorders. The aim of this study was to formulate a novel radiopharmaceutical for the detection of myocardial infarction at early stages in susceptible patients, which requires the development of high myocardium affinity radiopharmaceuticals able to establish an accurate in vivo quantification of cardiac β1-adrenoceptors. This was attained by the direct complexation of nebivolol as a cardioselective agent with technetium-99m as one of the most useful radionuclides in diagnostic nuclear medicine. Factors affecting the radiochemical yield such as nebivolol amount, stannous chloride amount, reaction time and pH of the reaction mixture were optimized. The results showed that the radiochemical yield was 95 ± 2.87 % and the radiolabeled compound was separated by high performance liquid chromatography. In vitro studies showed that the formed complex was stable for up to 24 h. In vivo uptake of 99mTc-nebivolol in the heart was 4.55 ± 0.23 % ID/g organ at 0.5 h post injection, whereas the clearance from Albino mice appeared to proceed via the hepatobiliary and renal clearance pathways. Predosing mice with cold nebivolol reduced the heart uptake to 1.1 ± 0.02 % and further confirmed the high specificity and selectivity of this radiotracer for the assessment of the myocardial β1-adrenoceptors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
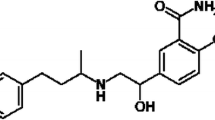
Nebivolol is a myocardial β1-selective adrenergic receptor antagonist that augments vascular nitric oxide release causing vasodilatory effects in humans. Nebivolol is chemically designated as 1-(6-fluorochroman-2-yl)-{[2-(6-fluorochroman-2-yl)-2-hydroxy-ethyl]amino}ethanol [1] (Fig. 1). In animal studies and cell systems, nebivolol was reported to be highly selective for β1-adrenoceptors [2–4]. The binding studies in human myocardium have revealed that nebivolol exhibits high affinity and specificity for the β1-adrenoceptor subtype (K i (β1): 6.1 nM) relative to the β2-adrenoceptor subtype (K i (β2): 149.7 nM) [5]. The β1-selectivity was studied in human myocardial tissue in comparison with several beta-blockers currently available for clinical use [6–8]. Nebivolol proved to be the most β1-selective adrenoceptor relative to other β1-selective antagonists such as bisoprolol, carvedilol, atenolol, metoprolol and betaxolol [9–12].
In the normal heart, mainly β1 and β2-ARs control the adrenergic functionality of the myocardium according to their molecular, biological, and pharmacological characteristics [13, 14]. The β1-adrenoceptor is the dominant receptor in heart, primarily responsible for the cardiac contraction and control of the heart rate [13]. On the other hand the failing human heart is characterized by a selective reduction in β1-adrenoceptors without change in β2-AR density. Changes in the β1/β2-AR ratio are associated with some cardiovascular diseases and disorders, such as heart failure, myocardial ischemia, myocardial infarction and hypertension [15–20]. Non-invasive quantification of β-ARs could facilitate the accurate choice and control of therapeutic interventions for patients with cardiovascular diseases [15, 16]. Consequently, the in vivo quantification of cardiac β1-adrenoceptors could become an independent prognostic marker for chronic heart failure and cardiovascular disorders [21, 22].
Several non-selective β1-AR radioligands have been used with both SPECT and PET molecular imaging techniques for cardiac imaging [23–36]. Radioiodinated derivatives of the non-selective β-AR antagonists carazolol and CGP-12177 were synthesized for quantifying β-ARs with SPECT in patients with heart disease [23–25]. Other radioligands such as [11C]CGP-12388 [26], [18F]CGP-12388 [27], [11C]CGP-12177 [28], have been used in PET studies. On the other hand, a few β1-AR selective radioligands, such as [11C]bisoprolol [29], [11C]HX-CH 44 [30], [11C]CGP-26505 [31], [11C]CGP-20712A [32] and 125I-nebivolol [33] were assessed in vivo. Quantification of β1-ARs with either SPECT or PET in patients with heart disease requires a radioligand with high affinity, specificity and low metabolism for clinical studies [34, 35]. At present none of the β1-selective radioligand turned out to be suitable for the non-invasive assessment of cardiac β1-ARs [34, 36].
The present study describes the development of a selective radioligand for the non-invasive assessment of cardiac ARs in vivo, so that their distribution, concentration and occupancy by endogenous ligand or drugs can be monitored throughout the progress of specific diseases and their treatments. This could identify patients who are at risk for future cardiovascular diseases and disorders.
Materials and methods
Nebivolol, M.wt. = 405.435 g/mol was a generous gift from Pharmagene Lab., Egypt. Whatman No. 1 paper chromatography (PC), Whatman International Ltd, Maidstone, Kent, UK. Technetium-99m was eluted as 99mTcO4 − from 99Mo/99mTc generator, Gentech, Turkey. The radioactivity was measured in a well-type NaI(Tl) crystal coupled to SR-7 scaler ratemeter. All Chemicals were of analytical or clinical grade and were used directly without further purification unless otherwise stated. Deionized water was used in all experiments for the preparation of solutions, dilution and washing purposes.
Labeling of nebivolol
99mTc-nebivolol was generally synthesized by direct reaction of nebivolol with 99mTc (t 1/2 = 6 h) under reducing conditions in the presence of SnCl2·2H2O. One ml of 99mTc eluate containing 195 MBq was added to the above mixture and left to react at room temperature (25 °C) for the recommended time before estimating the yield of 99mTc-nebivolol complex. The influence of various reaction parameters and conditions on radiolabeling efficiency, such as the amount of reducing agent (SnCl2·2H2O), concentration of nebivolol (50–225 μg), pH of the reaction medium (2–12) and the reaction time (1–480 min) was investigated and optimized in order to maximize the radiochemical yield. The radiochemical purity was assessed by PC and high performance liquid chromatography (HPLC).
Determination of radiochemical purity
Radiochemical purity of 99mTc-nebivolol was performed by paper chromatographic technique using strips of Whatman No. 1 paper. On two PC strips (1 cm width, 13 cm length), 1–2 μl of the reaction mixture was placed 2 cm above the lower edge and allowed to evaporate spontaneously. Then one strip was developed with acetone from which the percent free 99mTcO4 − was determined while the other strip was developed with a mixture of ethanol:water:ammonium hydroxide (2:5:1) from which the percent reduced hydrolyzed 99mTc was determined.
The radiochemical purity was further confirmed by HPLC (Hitachi model, Japan) The HPLC analysis of 99mTc-nebivolol complex was done by injection of purified 10 μl 99mTc-nebivolol complex into the column (Alphabond RP-C18, 300 × 3.9 mm) and UV spectrophotometer detector (SPD-6A) operated at 282 nm. The mobile phase consisting of a mixture of acetonitrile and 0.3 M potassium dihydrogen phosphate adjusted to pH 3.2 in the ratio of 50:50 v/v was delivered at a flow rate of 1.2 ml/min [37].
Stability in serum
Stability of 99mTc-nebivolol complex in serum was studied in vitro by mixing 1 ml of normal serum and 0.5 ml 99mTc-nebivolol and incubated at 37 °C for 24 h. Exactly 0.2 ml aliquots were withdrawn during the incubation at different time intervals up to 24 h and subjected to PC to determine the percent of 99mTc-nebivolol and free pertechnetate. Consequently, the stability of the radiolabeled complex will determine its suitability for in vivo application [38].
Biodistribution studies
The experimental procedures of the animal studies were in accordance with the guidelines set out by the Egyptian Atomic Energy Authority and were approved by the animal ethics committee, Labeled Compound Department. In vivo experiments were carried out in normal Albino mice (n = 5). Animals were injected intravenously in the tail vein with 99mTc-nebivolol (0.3 MBq/g). Animals were euthanized after injection of the tracer; Albino mice were sacrificed by cervical dislocation at 0.5, 2 and 4 h after administration of 99mTc-nebivolol. A blood sample was obtained by heart puncture.
After dissection, the organs and tissues were rinsed with saline, collected in plastic containers and weighed. The radioactivity of each sample as well as the background was counted in a well-type NaI(Tl) crystal coupled to SR-7 scaler ratemeter. Percent-injected dose per gram organ (% ID/g organ ± SD) in a population of five mice for each time point was reported. Blocking study, in which cold nebivolol was administered 30 min prior to the injection of 99mTc-nebivolol was reported as average uptake percent at 0.5 h post injection (n = 5). Differences in the data were evaluated with the Student t test. Results for P using the two-tailed test were reported and all results are given as mean ± SEM. The level of significance was set at P < 0.05.
Results and discussion
Radiochemical purity of a radiopharmaceutical product is the proportion of the total radioactivity in the desired radiochemical form. Radiochemical purity and in vitro stability of 99mTc-nebivolol complex were assessed by PC and HPLC. In PC using acetone as the developing solvent, free 99mTcO4 − moved with the solvent front (R f = 1), while 99mTc-nebivolol and reduced hydrolyzed technetium remained at the origin. Reduced hydrolyzed technetium was determined by using a mixture of ethanol:water: ammonium hydroxide (2:5:1) as the developing solvent, where reduced hydrolyzed technetium remains at the origin (R f = 0) while other species migrate with the solvent front (R f = 1). In most radiopharmaceutical preparations, the major fraction of radioactivity is in the bound form. The free and hydrolyzed fractions are undesirable radiochemical species and must be removed or reduced to a minimum level in order not to interfere significantly with the diagnosis. The radiochemical purity was determined by subtracting the sum of the percent of reduced hydrolyzed technetium and free pertechnetate from 100 %. The radiochemical yield is the mean value of three experiments.
The radiochemical purity was further confirmed by HPLC analysis, where the retention time of free 99mTcO4 − and 99mTc-nebivolol was 2.3 and 3.7 min, respectively as shown in the chromatogram (Fig. 2). The HPLC system was able to separate 99mTc-nebivolol and can be used for purification and quality control of the compound. Factors affecting the radiochemical yield will be discussed in details.
Effect of nebivolol amount
Nebivolol was labeled with technetium-99m using the direct technique, in which the reduced technetium-99m react with nebivolol to form the labeled chelate. The dependence of radiochemical yield on the amount of nebivolol was depicted in Fig. 3. The reaction was performed at different nebivolol concentrations (50–225 μg). Exactly 200 μg was the optimum ligand amount required to obtain maximum radiochemical yield, 95 ± 2.87 %. Below this value, the ligand amount was insufficient to complex all the reduced technetium-99m, as a result the amount of the reduced hydrolyzed technetium was high and was equal to 69 % at 50 μg of nebivolol. At ligand amount higher than 200 μg, the labeling yield remained stable (~95 %).
Radiochemical yield of 99mTc-nebivolol as a function of different amounts of nebivolol. Effect of nebivolol amount on the labeling yield of 99mTc-nebivolol; reaction conditions: x μg nebivolol, 200 μg SnCl2·2H2O, 1.0 ml (~195 MBq) of 99mTcO4 − at pH 6, the reaction mixture was kept at room temperature for 30 min
Effect of SnCl2·2H2O content
The effect of reducing agent (stannous chloride) on the labeling efficiency of 99mTc-nebivolol is demonstrated in Fig. 4. The radiochemical yield was dependent on the amount of SnCl2·2H2O present in the reaction mixture. At 10 μg SnCl2·2H2O, the labeling yield of 99mTc-nebivolol was 73 % due to incomplete reduction of 99mTcO4 − and hence unreliable yield of the complex due to the presence of free 99mTcO4 − (13 %). The labeling yield was significantly increased by increasing the amount of SnCl2·2H2O from 10 to 200, at which a maximum labeling yield of 95 ± 2.87 % was obtained. By increasing the amount of SnCl2·2H2O above the optimum concentration value, the labeling yield decreased (65 %) due to the formation of tin colloids (35 %), which can compete with nebivolol for the reduced 99mTc.
Radiochemical yield of 99mTc-nebivolol as a function of reducing agent amount. Effect of SnCl2·2H2O concentration on the labeling yield of 99mTc-nebivolol; reaction conditions: 200 μg nebivolol, x μg of SnCl2·2H2O, 1.0 ml (~195 MBq) of 99mTcO4 − at pH 6, the reaction mixture was kept at room temperature for 30 min
Effect of reaction time and in vitro stability
The labeling yield of nebivolol with 99mTc is strongly dependent on reaction time in the range from 1 to 480 min. The effect of time on the in vitro stability of 99mTc-nebivolol complex was studied in order to determine the suitable time during which the preparation can be used. It is clear from Fig. 5 that the radiochemical yield was significantly increased when increasing the reaction time from 1 to 30 min. Increasing the reaction time beyond 30 min caused slightly decrease in the radiochemical yield. A minimum reaction time of 30 min was needed to reach the maximum radiochemical yield (95 ± 2.87 %). The labeled complex was stable for up to 8 h after labeling.
Effect of pH
The influence of the pH of the reaction mixture on the radiochemical yield of 99mTc-nebivolol is shown in Fig. 6. The pH of the reaction medium was studied at pH range from 2 to 12. The radiochemical purity of the preparation is highest at pH 6 and was equal to 95 ± 2.87 %. At lower pH (<6), the reducing power of Sn(II) ion works strongly in acidic medium to reduce technetium to lower oxidation state, which favors the formation of 99mTc-nebivolol. At pH above 6 the radiochemical purity was significantly lowered due to the formation of reduced hydrolyzed 99mTc, which is the main radiochemical impurity at pH 12.
Biodistribution studies
Biodistribution studies of 99mTc-nebivolol is shown in Table 1. All radioactivity levels are expressed as average percent-injected dose per gram body organs (% ID/g organ ± SD). The biodistribution data of 99mTc-nebivolol showed an uptake of 4.533 ± 0.021 % ID/g organ in the cardiac muscle at 0.5 h post injection (pi). After this time point radioactivity dropped to 1.273 ± 0.006 % at 2 h pi and 0.516 ± 0.010 % at 4 h pi.
The biodistribution results demonstrated that the complex was cleared efficiently from the blood stream with only 0.972 % remaining in the blood after 4 h postinjection in comparison to 4.969–10.895 % at 2 and 0.5 h post injection, respectively. The early hepatic uptake was relatively higher (21.255 ± 0.023 %) than the renal uptake (17.764 ± 0.012 %), which reflected that the clearance pathways of 99mTc-nebivolol from the mice appeared to proceed via the hepatobiliary and renal clearance pathways. The level of radioactivity in all organs rapidly dropped between 2 and 4 h pi uptake of 99mTc-nebivolol except for stomach (3.193 ± 0.201 %), which was observed slightly increasing with time.
Predosing Albino mice with non radioactive β1-AR nebivolol 0.5 h before the injection of 99mTc-nebivolol reduced the heart uptake to 1.1 ± 0.022 % ID/g organ at 0.5 h pi. This result suggested that 99mTc-nebivolol binds selectively to β1-ARs in the heart and that the uptake was specific. As a result of this study, 99mTc-nebivolol can be used successfully in imaging β1-adrenoceptors.
Conclusions
Nebivolol was labeled with 99mTc by direct labeling technique with a high labeling yield of 95 ± 2.87 % using SnCl2·2H2O as reducing agent. This study described the in vitro and in vivo characterization of 99mTc-nebivolol necessary for designing a potentially useful radiopharmaceutical for the β1-adrenergic receptor imaging. It showed good radiochemical and metabolic stability in vivo. Biodistribution studies performed in Albino mice demonstrated the affinity and specificity of 99mTc-nebivolol for β1-adrenergic receptors.
References
Indian Pharmacopoeia (2010) vol 3. New Delhi, p 1753
Pawels PJ, Gommery W, Van Lommen G, Janssen PAJ, Leysen JE (1989) Mol Pharmacol 34:843
Pawels PJ, Gommery W, Van Gompel P, Leysen JE (1991) Biochem Pharmacol 42:1683
The NIH Website. http://dailymed.nlm.nih.gov/dailymed/. Accessed 10 2008
Bundkirchena A, Nguyena Q, Brixiusa K, Bölck B, Mehlhorn U, Schwingera RHG (2003) Eur J Pharmacol 476:97
Brixiusa K, Bundkirchena A, Bölck B, Mehlhorn U, Schwinger RHG (2001) Br J Pharmacol 133:1330
Maack C, Tyroller S, Schnabel P, Cremers B, Dabew E, SuÈdkamp M, BoÈhm M (2001) Br J Pharmacol 132:1817
Mangrella M, Rossi F, Fici F (1998) Pharmacol Res 38:419
Münzel T, Gori T (2009) J Am Coll Cardiol 54:16
Schnabel P, Maack C, Mies F, Tyroller S, Scheer A, Böhm M (2000) J Cardiovasc Pharmacol 36:466
Smith C, Teitler M (1999) Cardiovasc Drugs Ther 13:123
Cheng YC, Prussoff WH (1973) Biochem Pharmacol 22:3099
Sarsero D, Molenaar P, Kaumann AJ, Freestone NS (1999) Br J Pharmacol 128:1445
Brodde OE, Michel MC (1999) Pharmacol Rev 51:651
Molenaar P, Parsonage WA (2005) Trends Pharmacol Sci 26:368
Brodde OE, Bruck H, Leineweber K (2006) J Pharmacol Sci 100:323
Castellano M, Böhm M (1997) Hypertension 29:715
Yamada S, Ohkura T, Uchida S, Inabe K, Iwatani Y, Kimura R, Hoshino T, Kaburagi T (1996) Life Sci 58:1737
Khamssi M, Brodde OEJ (1996) Cardiovasc Pharmacol 16(Suppl 5):S133
Brodde OE, Zerkowski HR, Doetsch N, Motomura S, Khamssi M, Michel MC (1989) J Am Coll Cardiol 14:323
Anthonio RL, Brodde OE, van Veldhuisen DJ, Scholtens E, Crijns HJ, van Gilst WH (2000) Int J Cardiol 72:137
Schäfers M, Dutka D, Rhodes CG, Lammertsma AA, Hermansen F, Schober O, Camici PG (1998) Circ Res 82:57
Dubois EA, van den Bos JC, Doornbos T, van Doremalen PA, Somsen GA, Vekemans JA, Janssen AG, Batink HD, Boer GJ, Pfaffendorf M, van Royen EA, van Zwieten PA (1996) J Med Chem 39:3256
van den Bos C, van Doremalen PA, Dubois E, Somsen GA, Vekemans JA, Janssen AG, Boer GJ, Pfaffendorf M, van Royen EA, van Zwieten PA (1997) Nucl Med Biol 24:1
Dubois EA, Somsen GA, van den Bos JC, Janssen AG, Batink HD, Boer GJ, van Royen EA, van Zwieten PA (1997) Nucl Med Biol 24:9
Elsigna PH, van Waarde A, Jaeggi KA, Schreiber G, Heldoorn M, Vaalburg W (1997) J Med Chem 40:3829
de Jong RM, Willemsen ATM, Slart RHJA, Blanksma PK, van Waarde A (2005) Eur J Cardiovasc Nurs 4:99
Delforge J, Syrota A, Lancon JP, Nakajima K, Loch C, Janier M, Vallois JM, Cayla J, Crouzel C (1991) J Nucl Med 32:739
Soloviev DV, Matarrese M, Moresco RM, Todde S, Buonasera TA, Sudati F, Simonelli P, Magni F, Colombo D, Carpinelli A, Kienle MG, Fazio F (2001) Neurochem Int 38:169
Valette H, Dolle F, Guenther I, Demphel S, Rasetti C, Hinnen F, Fuseau C, Crouzel C (1999) Nucl Med Biol 26:105
van Waarde A, Meeder JG, Blanksma PK, Brodde OE, Visser GM, Elsinga PH, Paans AMJ, Vaalburg W, Lie KI (1992) Eur J Pharmacol 1:107
Elsigna PH, van Waarde A, Visser GM, Vaalburg W (1994) Nucl Med Biol 21:207
Motaleb MA, Moustapha ME, Ibrahim IT (2011) JRNC 289:239–245
Law MP, Wagner S, Kopka K, Rennera C, Pike VW, Schober O, Schäfers M (2010) Nucl Med Biol 37:517
Pike VW, Law MP, Osman S, Davenport RJ, Rimordi O, Giardina D, Camici PG (2000) Pharm Acta Helv 74:191
Kopka K, Law MP, Breyholz HJ, Faust A, Höltke C, Riemann B, Schober O (2005) Curr Med Chem 12:2057
Gupta Y, Shrivastava A, Duggal D, Patel A, Agrawal S (2009) J Young Pharmacists 1:264
Moustapha ME, Motaleb MA, Ibrahim IT (2011) JRNC 287:1
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakr, T.M., Moustapha, M.E. & Motaleb, M.A. 99mTc-nebivolol as a novel heart imaging radiopharmaceutical for myocardial infarction assessment. J Radioanal Nucl Chem 295, 1511–1516 (2013). https://doi.org/10.1007/s10967-012-2168-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-012-2168-1