Abstract
The monitoring of long-lived radionuclides is of great importance in the context of the surveillance of nuclear facilities, during their operation as well as during their decommissioning. This is especially true for radionuclides of rather volatile elements, such as chlorine and iodine, the main interest being in 36Cl and 129I. Liquid Scintillation Counting (LSC) is a widely used measurement technique especially for the determination of 36Cl that requires a thorough and selective sample preparation in order to give accurate results. Sample preparation methods frequently employed such as volatilization and/or repeated precipitation steps can be rather elaborate and time consuming. Therefore, an attempt has been made to develop an ‘easy to use’ extraction chromatographic resin that allows extraction, and subsequent separation, of chloride and iodide from pretreated environmental and decommissioning samples for their determination via LSC. First results of the characterization of the resin including D w values of Cl−, I− and potential interferents, and of the method development are presented as well as the result of the analysis of a simulated real sample.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Beside numerous other radionuclides, the long-lived and rather volatile 36Cl (half-life 3.01(3) × 105 years; 98.1% β− decay to 36Ar, E β,max: 708.6 keV; 1.9% electron capture to 36S [1]) and 129I (half-life 16.1(7) × 106 years; 100% β− decay to 129Xe, E β,max: 190.8 keV [1]) are determined in various sample matrices within the context of the monitoring of nuclear installations, during operation and especially also during decommissioning. 36Cl is, in addition to natural production processes, produced in nuclear installation during the irradiation of nuclear fuel by neutron activation of 35Cl [2]. 129I is a fission product that is, compared to 131I (half-life 8.0233(19) d [1]), produced with a rather low yield, but that has a very long half-life [3]. Both, 36Cl and 129I can be released as gaseous and/or liquid effluents and will be present in radioactive wastes originating from nuclear installations.
Numerous techniques for the determination of 36Cl and 129I are described in literature, 36Cl is frequently determined via AMS [4, 5] or LSC [2, 6, 7], 129I via NAA [8], AMS [9], ICP-MS [10, 11] or ß-spectrometry [12]. LSC measurements have the advantage of availability for a large number of laboratories and are thus interesting especially for routine monitoring. In order to allow the determination of 36Cl and 129I via LSC, and to obtain accurate and precise results, the samples have to pass a number of sample preparation steps; among these steps are the chemical separation and purification of the nuclides and the subsequent preparation of counting samples suitable for LSC. Sample preparation methods frequently employed such as volatilization [2, 6, 12], precipitation and/or ion exchange steps [7] can be rather elaborate. Therefore, the attempt of developing an extraction chromatographic resin that allows extraction, and subsequent separation, of Cl− and I− from pretreated environmental and decommissioning samples has been made. The suggested method only applies to chloride and iodide, any chlorine or iodine present in the sample in elemental form or as oxoanions will need to be reduced to chloride or iodide during sample preparation.
Results of the characterization of the resin are presented as well as some first results of the development and validation of methods based on this resin. The results of the analysis of a simulated real sample are presented.
Experimental
Reagents and apparatus
All reagents used were of analytical grade, 18 MΩ deionized water was used throughout all experiments. pH values were measured using a pH meter and adjusted when necessary using NaOH and H2SO4. The Cl resin samples prepared by TrisKem International were used as received without further purification. Ag+ loaded Cl resin was prepared by contacting 10 g of the Cl resin with 100 mL of a 1 M H2SO4 solution containing 650 mg of AgNO3 in a 250 mL PE flask on a vortex shaker over a period of 2 h. The resin was then filtered, rinsed with 1 M H2SO4 (until absence of Ag+ in the filtrate) and dried.
2 mL PP columns with appropriate funnels (both TrisKem International) were employed for elution studies and separation tests, 2 mL Eppendorf centrifuge tubes for batch experiments. Element concentrations were determined by ICP-MS measurement using an ELAN 6000 (PerkinElmer), all samples were measured after appropriate dilution and addition of a suited internal standard. LSC measurements were performed using either a TriCarb 1600TR (Packard) or a 1220 Quantulus (PerkinElmer) and ProSafe HC (Meridian) as liquid scintillation cocktail.
Determination of weight distribution ratios D w
For Ag and selected elements weight distribution ratios were determined. 50 mg of the Cl resin were weighted into 2 mL centrifugation tubes and 0.3 mL of the acid for which the D w was to be determined was added. The tubes were closed and shaken for 30 min in order to precondition the resin. 1 mL of a solution of a well defined pH value, containing defined amounts of selected cations, in general 1 μg per cation, was then added (A0 sample). The tubes were again closed, shaken for 30 min and finally centrifuged. A 1 mL aliquot was withdrawn from the tube using a micro-pipette (A sample). The aliquot was diluted appropriately using 3% HNO3 and measured by ICP-MS. Measurements were performed relative to the A0 sample.
I− and Cl− weight distribution ratios were determined using Ag+ loaded Cl resin prepared as described before and A0 samples containing known activities of 36Cl or 129I. Conditions tested included 1 M H2SO4 as well as varying KSCN (0.01–0.2 M) and Na2S (0.04–0.35 M) concentrations. The withdrawn aliquots were mixed with 10 mL of liquid scintillation cocktail and measured by liquid scintillation counting (counting time t = 10 min) relative to A0 samples. All D w values were determined in triplicate.
Dw values were calculated according to Eq. 1, net count rates refer to ICP-MS count rates as well as to LSC count rates:
where NA0 = net count rate in the A0 sample; NA = net count rate in the A sample; V = Volume of the aqueous phase (1.3 mL) and mR = amount of the resin in g.
Maximum uptake
First, the Ag+ uptake of the Cl resin was examined in batch experiment. 50 mg of the resin were preconditioned as described before and contacted in a 2 mL centrifuge tube with 1 mL of a Ag+ standard solution (A0 sample; 1 M H2SO4 containing 5 mg Ag+ mL−1) under shaking for 30 min. A 1 mL aliquot was withdrawn using a micro-pipette. The aliquot was diluted appropriately using 3% HNO3 and measured by ICP-MS. Measurements were performed relative to the A0 sample. The Ag+ uptake (K) of the resin was calculated according to Eq. 2:
where c Ag,A0 = silver concentration in the A0 sample, c Ag,A = silver concentration in the A sample, V A0 = volume of the A0 sample, V A = volume of the A sample, m R = mass of the resin in g.
Additionally the silver uptake was also examined by column elution experiments. Several columns were prepared in order to allow estimating the influence of the contact time on the Ag+ uptake. The columns used for the uptake studies were prepared by soaking 0.65 g of Cl resin in 10 mL 1 M H2SO4 for 2 h under shaking; the resin was subsequently transferred into an empty 2 mL column. The packed columns were then loaded with a small volume (2 mL) of a 1 M H2SO4 silver nitrate solution with a well known Ag+ concentration (10 mg Ag+ mL−1) and allowed to stand for 0.5, 2.5, 10 h, respectively. The columns were then rinsed three times with 10 mL 1 M H2SO4, the rinsing solutions were collected in a volumetric flask, appropriately diluted and analyzed by ICP-MS for Ag content.
Cl− and I− uptake behaviours were also examined via column experiments using Cl resin columns previously loaded with Ag+. In case of Cl− uptake, the columns were loaded with 5 mg of Cl−, in case of I− with 20 mg of I− (both as sodium salts). Loaded columns were rinsed three times with 1 M H2SO4, all rinsing solutions were collected, diluted and analyzed by ICP-MS. Calculations were performed analogous to Eq. 2, obtained experimental results were compared to theoretical values (quantitative precipitation of I−/Cl– by 13 mg of Ag+).
Elution studies
The columns used during the elution studies were prepared similarly as described above. The resin was soaked in 1 M H2SO4 for 2 h and then transferred into the column. The columns were then loaded with 2 mL of 1 M H2SO4 solution containing 13 mg of Ag+ and allowed to stand for 2.5 h. The main aim of the elution study was to determine optimum volumes of the respective rinsing and elution steps. The loading solutions were collected directly, whereas rinsing and elution steps were collected in 5 mL fractions.
Elution studies on Cl− and I− were performed using sample load solutions that were 1 M in H2SO4 and that contained either 50 Bq 36Cl and 1 mg of Cl− (as sodium chloride) or 50 Bq 129I and 1 mg of I− (as sodium iodide). The elution studies were first performed separately for Cl− and I−. Elution conditions evaluated included 0.1 M KSCN (for Cl− elution) and 0.35 M Na2S (for I− elution), both at pH 7, as well as several different rinsing steps upfront to the I− elution with the focus of evaluating their influence on the I− elution yield.
The best suited elution conditions obtained from the elution studies were applied to a loading solution containing known activities of both, 36Cl and 129I, in order to confirm its suitability for the foreseen separation.
Decontamination factors
Data obtained during the Cl−/I− elution studies using the optimized method were also used to calculate decontamination factors (D f) for Cl− and I− in the respective I− and Cl− fractions. These were calculated according to Eq. 3:
where N 0 = net count rate of the element in the A0 sample; N a = net count rate of the element in the respective fraction.
In case the count rate in the respective fraction was lower than the decision level (DL), the decontamination factor D f was calculated according to Eq. 4
where DL is calculated for LSC measurements using Eq. 5:
wherein: k 1−α and k 1−β = quintiles of the normal distribution, N 0 = background count rate, t 0 = background counting time and t m = sample counting time.
The optimized elution conditions and volumes were further applied to loading solutions containing a number of different elements (Cr, Mn, Co, Ni, Cu, Zn, Rb, Sr, Cd, Cs, Ba, Pb, U; all 10 μg in 10 mL loading solution). Cl− and I− fractions were collected and analyzed by ICP-MS; decontamination factors for these elements were calculated as indicated above, in case the concentration of an elements was below the decision limit of the ICP-MS the decontamination factors were calculated according to Eq. 4 with DL being three times the standard deviation of the background count rate of the element in the blank sample.
Finally loading solutions containing known activities of 90Sr/90Y, 60Co or 137Cs were prepared and separated on the Cl resin columns, chloride and iodide fractions were collected and analyzed by LSC, decontamination factors were calculated according to Eqs. 3 and 4.
Precision
In order to get some first information on the precision of the method a 300 mL tap water sample was spiked with 36Cl and 129I (activity level of the final sample: each 0.5 Bq mL−1); homogenized and divided into 30 subsamples. The samples were analyzed over 3 days, 10 samples per day. Standard deviations were calculated according to Eqs. 6–8, wherein the total combined standard deviation s t was calculated from the intra-day standard deviation s b and the inter-day standard deviation s w.
Simulated real sample
A simulated real sample was prepared by adding well known activities of 36Cl (47.2 ± 2.4 Bq) and 129I (41.1 ± 3.1 Bq), as well as known activity levels of 60Co, 90Sr/90Y and 137Cs (each 85 Bq) to 50 mL of a tap water sample previously adjusted to 1 M H2SO4. 10 mL aliquots of this solution were analyzed in triplicate following the optimized separation method, and the Cl− and I− fractions were analyzed by LSC. Mean yields and contributions of the precision to the overall uncertainty were taken from the results of the precision experiments and used for activity and uncertainty calculation. Results were evaluated according to Eqs. 9 and 10.
wherein U = u c × k with k = 2, A R = reference activity, A D = determined activity.
Results and discussion
Weight distribution ratios D w
In order to evaluate the suitability of the resin for the foreseen use, the D w of Ag+ was first determined for a number of acids and acid concentrations; however, for practical reasons the main focus was set on sulfuric acid. The D w values of Ag+ in H2SO4 on the Cl resin were found to be: 65,000 mL g−1 in 1 M H2SO4, 60,000 mL g−1 at pH 3 and 35,000 at pH 5. Ag+ is thus very well extracted over a wide range of pH values with a maximum in 1 M H2SO4. At this concentration the resin has, as Table 1 shows, high selectivity for Ag and Pd whereas none of the other elements tested show relevantly high D w values. The resin’s selectivity is thus overall suited for the purpose.
Dw values were determined for Cl− and I− in 1 M H2SO4, in 0.01, 0.05, 0.1 and 0.2 M KSCN; in addition I−Dw values were determined in 0.04, 0.09, 0.18 and 0.35 M Na2S. For both Cl− and I−, the Dw values in 1 M H2SO4 were greater than 1,000 (Cl: 1,600, I: 2,000). These conditions thus allow not only excellent Ag+ retention, but also very good chloride and iodide uptake. Accordingly 1 M H2SO4 was chosen as sample loading condition for all further testing. Figures 2 and 3 summarize the Dw values obtained for Cl− and I− under varying KSCN and Na2S concentrations. Figure 1 shows that even small amounts of KSCN interfere very strongly with the Cl− uptake whereas I− remains very well retained. The Cl−/I− separation seems thus easily possible using a dilute KSCN solution. For further testing 0.1 M KSCN was used. I− can, as Fig. 2 shows, be eluted with Na2S solutions of elevated concentration. For further testing 0.35 M was chosen.
Maximum uptake
The Ag+ uptake of the Cl resin was determined to be 38.5 ± 1.3 mg Ag+ g−1 resin under the described batch extraction conditions. Ag+ uptake in column geometry was found to be lower under the chosen conditions; additionally it was found that the uptake is time dependent: after 30 min 16.5 ± 0.8 mg of Ag+ were extracted, after 2.5 h 19.5 ± 1.0 mg of Ag+. Longer contact times did not result in a further increase of the Ag+ uptake. For all further experiments, the resin was contacted with the Ag+ for about 2 h in order to assure maximum uptake, for routine use a time of 30 min would seem sufficient.
The Cl− and I− uptake of the Cl resin under the chosen conditions were found to be 4.3 ± 0.2 mg Cl− g−1 resin and 16.3 ± 1.6 mg I− g−1 resin, which agrees well with the theoretical values of 4.2 mg Cl− g−1 resin and 14.9 mg I− g−1 resin.
Elution studies
Figures 3 and 4 show the results of the initial elution studies; Fig. 3 shows that 5 mL 0.1 M KSCN quantitatively remove Cl− from the column, whereas, as Fig. 4 shows, no I− is eluted; it further shows that I− is mainly eluted in the first 5 mL of 0.35 M Na2S, almost none being eluted in the later fractions. Nevertheless, the I− yield was found to be only in the order of 70%. Follow up studies were performed using various additional rinsing steps between the Cl− and the I− elution step including increasing amounts of deionized water, reducing and oxidizing agents and alkaline solutions. Figure 5 shows the I− yield in the I− fraction in function of the rinsing step performed before the elution. 1% NH3 and 1% NaOH solutions proved to be the best choice, resulting in near quantitative I− elution. For practical reasons 1% NaOH was retained as additional rinsing step, since NH3 complexed and eluted parts of the Ag+ retained on the Cl resin column. The best suited separation method can thus be summarized as follows:
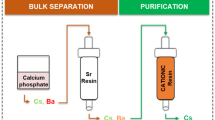
The sample loading solutions are adjusted to 1 M H2SO4 (in order to assure that iodine and chlorine are present as chloride and iodide a strong reducing agent, e.g. Sn(II) might be added to the sample loading solution) and loaded onto the Ag+ loaded Cl resin column. The column loading is followed by a first rinsing step with 10 mL deionized water in order to increase the pH on the column and to remove matrix elements and potential interferents. Cl− is eluted with 5 mL 0.1 M KSCN. The column is then further rinsed with 10 mL 1% NaOH in order to optimize the I− elution and finally I− is eluted using 5 mL 0.35 M Na2S. Figure 6 summarizes the method.
In order to verify that the Cl−/I− separation is working sufficiently well for the foreseen use a solution containing 36Cl and 129I was separated using the described method, the observed separation is very clean, as Fig. 7 shows.
Decontamination factors
The determined decontamination factors D f are summarized in Table 2. In all cases the net count rates in the Cl− and I− fractions were below the respective decision limits, accordingly all D f values are given as being “greater than”. Overall the results indicate that the Cl resin and the chosen separation method show a suitable selectivity for the intended use. These results will have to be confirmed by the analysis of real samples.
Precision
Table 3 shows the results of the precision experiments. The Cl− elution shows very good precision with a s t < 3%. The precision of the I− elution on the other hand is acceptable, but with a st in the order of 9% significantly lower than the Cl− precision. In any case it is advisable to use stable Cl− and I− as internal standards.
Simulated real samples
Table 4 compares the results obtained experimentally with the expected results. The determined and reference activities agree well, because the E n values are below 1. The determined results are thus not deviating significantly from the expected ones. The bias between determined and expected results is well below 10%, nevertheless the 36Cl results show a slight negative bias, the use of an internal standard, for 36Cl as well as for 129I, is thus advisable. The results are regrouped rather well, the sb value is 2.5% for 129I results (number of replicates N = 3) and 0.95% for 36Cl results (N = 3), further confirming the good precision of the method.
Conclusions and outlook
An extraction chromatographic resin showing very good selectivity for silver and platinum group metals was characterized and tested with respect to its use as a Cl selective resin after loading with Ag+. The resin showed overall good Cl− and I− uptake under loading conditions. Elution conditions were identified allowing separate elution of Cl− and I−. Through elution studies the best suited separation conditions and elution volumes were identified. The developed method was tested for 36Cl and 129I containing solutions, as well as for solutions containing numerous elements/potential interferents in order to get information on respective decontamination factors. Results indicated a good selectivity of the method and no major interferent could be identified. The method showed, at least for water samples, a very good precision for the Cl− separation, and an acceptable precision for the I− separation, nevertheless the use of internal standards is advisable. The analysis of a simulated real sample showed good agreement between obtained and expected results, though a slight negative bias was observed in the case of 36Cl. Results obtained so far indicate that the resin and the method have a good potential towards the foreseen use. Further development of the method with respect to the analysis of environmental and decommissioning samples (e.g. filter, soil and concrete samples) will be performed in the future. The suggested method will only allow the extraction and determination of iodine and chlorine in I− and Cl− form, oxoanions of these elements present in the sample solutions will thus have to be reduced before the separation in order to be also extracted; this fact will be addressed in further experiments. The so developed methods will then be validated with respect to precision, linearity, and especially accuracy by analyzing real samples in comparison to already validated methods and by analyzing reference materials.
References
LNHB Recommended data (2010) Table of radionuclides. http://www.nucleide.org/DDEP_WG/DDEPdata.htm. Accessed 20 Apr 2010
Rodriguez M, Pina G, Lara E (2006) Radiochemical analysis of Chlorine-36. Czechoslov J Phys 56(Suppl D):211–217
IRSN I-129 information sheet (2010). Fiche Radionuclide I-129 et environment. http://www.irsn.fr/FR/larecherche/Information_scientifique/Publications_Documentation/fiches-techniques-radionucleides/environnement/Documents/Iode_I129_v1.pdf. Accessed 20 Apr 2010
Cecil DL et al (2000) Use of chlorine-36 to determine regional-scale aquifer dispersivity, eastern Snake River Plain aquifer, Idaho/USA. Nucl Instrum Meth Phys Res B 172(1–4):679–687
Baxter M et al (2009) A sensitive method for the determination of chlorine-36 in foods using accelerator mass spectrometry. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 26(1):139–144
Itoh M et al (2002) Determination of 36Cl in biological shield concrete using pyrohydrolysis and liquid scintillation counting. Analyst 127(7):964–966
Hou Xiaolin, Østergaard LF, Nielsen SP (2007) Determination of 36Cl in nuclear waste from reactor decommissioning. Anal Chem 79(8):3126–3134
Muramatsu Y, Uchida S, Ohmomo Y (1990) Determination of I-129 and I-127 in soil and tracer experiments on the adsorption of iodine. J Radioanal Nucl Chem 138(2):377–384
Schmidt A et al (1998) On the analysis of iodine-129 and iodine-127 in environmental materials by accelerator mass spectrometry and ion chromatography. Sci Total Environ 223(2–3):131–156
Bienvenu P et al (2004) Determination of iodine 129 by ICP-QMS in environmental samples. Can J Anal Sci Spectrosc 49(6):423–428
Yoshida S et al (2007) Determination of the chemical forms of iodine with IC-ICP-MS and its application to environmental samples. J Radioanal Nucl Chem 273(1):211–214
Kabai E, Vajda N, Gaca P (2003) Simultaneous determination of radioactive halogen isotopes and 99Tc. Czechoslov J Phys 53(Suppl 1):A181–A188
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zulauf, A., Happel, S., Mokili, M.B. et al. Characterization of an extraction chromatographic resin for the separation and determination of 36Cl and 129I. J Radioanal Nucl Chem 286, 539–546 (2010). https://doi.org/10.1007/s10967-010-0772-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-010-0772-5