Abstract
A purification method is developed to determine 90Sr in radioactive waste. A separation based on Sr-resin® with a pretreatment using TRU-resin® provides satisfactory Sr recovery yields before the 90Sr measurement by liquid scintillation counting (LSC) regardless the analyzed samples with low or high activity level. The selectivity of the procedure is checked by measuring the 90Y ingrowth after different days of separation without waiting for secular equilibrium. In order to obtain a REACH compliant method without scintillation cocktails, a plastic scintillation resin selective for Sr is implemented on the basis of the developed protocol. The optimized method is applied successfully to representative nuclear waste including samples with high Pu content (effluents, concretes and sludges).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many older facilities of the nuclear industry have shut down or are planned to be stopped and need to be dismantled. At the same time, numerous projects for new nuclear power plants are emerging to meet the world's growing demand for energy. As a result, the growth of decommissioning and dismantling activities combined with new reactor projects imply an increase of the demand for radioactive waste characterization [1]. Therefore, it is necessary to have efficient and fast analytical methods that are also compliant with safety and environmental regulations to determine the different radionuclides contained in nuclear waste.
90Sr is known for its high mobility in the environment such as its chemical analogue Ca, so it is of prime interest to measure it in radioactive waste. 90Sr is a typical fission product and a pure β emitter with an intermediate half-life (28.8 years) [2]. By disintegration, it becomes 90Y, a pure β emitter with a short period (2.67 days). The characterization of 90Sr in nuclear waste requires its separation from the matrix and its interferents prior to nuclear measurements, generally with proportional or gas flow counters or liquid scintillation counters (LSC).
Through the years, many radiochemical purification methods have been developed to analyze 90Sr accurately. The first one was based on a succession of selective precipitations, in particular with nitric acid [3, 4]. Despite its proven efficiency and some advantages, it is not the most popular method nowadays [5, 6]. Procedures using liquid–liquid extraction or ion exchange chromatography were widely implemented but never replaced the precipitation method [7,8,9]. In the most recent years, ICP-MS, especially with triple quadrupole detection, has become an interesting option to quantify 90Sr without chemical purification but due to its high detection limit and high price, it is used on a limited scale [10, 11].
Currently, methods based on extraction chromatographic resins selective for Sr, named Sr-resin® developed by Eichrom in the 1990’s and now commercialized by Triskem, are the most preferred methods by characterization laboratories [12,13,14]. The advantage of this type of resin is that it combines the selectivity of liquid–liquid extraction and the rapidity of liquid chromatography. Its practicality, its efficiency, and the absence of use of high-risk products make it a popular alternative. Horwitz et al. [14] demonstrated the high selectivity of Sr-resin® to remove the main interferents of Sr, for example Y and Ca. Concerning the separation steps on Sr-resin®, the different methods published are very similar with the implementation of nitric acid as eluent with some variations of concentrations and volumes used. Besides, it was highlighted that Pu behaves similarly to Sr when using nitric acid as eluent for Sr-resin®, which can be problematic for some fields of applications such as nuclear waste [14]. A second point of vigilance towards this resin concerns high concentrations (above 1 M) of Ca, Na, K, or ammonium nitrates in samples: in such conditions, the capacity of the column to retain Sr can be altered [13]. Therefore, it is essential to be careful depending on the samples analysed and a pretreatment step might be required prior to the Sr-resin® separation for complex samples such as nuclear waste.
In the literature, various types of pre-treatments have been investigated [6]. For example, in the french standard method dedicated to 90Sr measurement in nuclear waste, five options are presented depending on sample characteristics: carbonate, phosphate or sulphate precipitations, anion exchange resin or cation exchange resin with organic complexing agent [15]. For a simple water sample, a protocol without pretreatment can be applied [16] but for more complex samples, such as with tetravalent actinides, the addition of oxalic acid in the mobile phase is recommended during the separation on Sr-resin® [16]. Pre-treatments can include precipitations, co-precipitations, ion exchange chromatography and extraction chromatography (for example, with DGA® or TRU® resins) [17].
In our laboratory, a purification method based on ammonia and carbonate precipitations is implemented before the Sr-resin® separation for 90Sr determination in nuclear waste. It has been developed as an alternative procedure towards the method based on fuming nitric acid [5]. It has been motivated by the need to improve 90Sr sensitivity and to have an efficient way to treat samples with high Ca and Ba contents. Indeed, in our Sr-based protocol, 90Sr is measured by LSC and recovery yield based on stable Sr by ICP-AES. The use of LSC provides a sensitivity gain of about a factor of 4 because the counting efficiency increases from around 0.25 to 1 in comparison to proportional counter (for example, G5000 Traveler device from Gamma products) [5]. Moreover, the Sr recovery yield obtained by gravimetry for the method based on fuming nitric acid can be overestimated due to Ca and Ba which are not eliminated completely when they are present in high quantity. The Sr-based procedure gives satisfactory results for most applications but unexpected problems of recovery were observed. Sr recovery yield can be lower than 10% for some effluents or concretes. Consequently, improving this current method is necessary to have a procedure that can be applied to various types of radioactive waste with high recovery and accuracy.
In addition to considerations of efficiency and performance, purification methods must be compliant with safety regulations. At the end of our protocol, 90Sr is determined by LSC that implies the addition of a scintillation cocktail prior to its measurement. The current scintillation cocktails contains nonylphenol ethoxylates (NPE), a surfactant concerned by the European REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals) regulation [18]. Therefore, in a very near future, scintillation cocktails containing NPE will be prohibited in laboratories or, at the very least, will require authorization be used. The first solution to this problem is the use of NPE free scintillation cocktails [19] but there is little experience with these products and their counting efficiency can be lowered [20]. For this study, a second option has been preferred: plastic scintillation with the implementation of plastic scintillation resin selective for Sr, named Sr PS-resin in the present work.
Plastic scintillator can be described as solid solution of fluorescing agent in aromatic solvent polymers. Once optimized, plastic scintillators are equivalent to scintillation cocktails in terms of role and composition [21]. In 2004, a study proved the efficiency of plastic scintillation technique for measurement of beta emitters such as 90Sr [22]. Following these successful results, a scintillating extraction chromatographic resin was developed by the same group [23]. The extractant used for Sr-resin®, a crown ether namely 4,4(5)-di-t-butylcyclohexano-18-crown-6, was impregnated on the microspheres of plastic scintillator. The resin has a selectivity similar to the commercialized Sr-resin® [23]. When working with Sr PS-resin, purified 90Sr which is retained on the resin is not eluted contrary to what is done with Sr-resin® and is directly measured in a scintillation counter. The procedure based on plastic scintillation resin is compliant with REACH regulation by avoiding the use of scintillation cocktails. Moreover, since no elution step is carried out, this procedure saves time and reduces nuclear waste produced. The Sr PS-resin was validated on milk and environmental samples [24, 25] but has not yet been applied to radioactive wastes.
The goal of this study is to demonstrate the robustness and efficiency of a new pre-treatment for a radiochemical procedure based on Sr-resin® in order to determine 90Sr accurately in nuclear waste samples. The work investigates different ways to solve low recovery problems encountered for some samples with our reference method. The new method relies on the implementation of another extraction chromatographic resin prior to Sr-resin®: the TRU-resin® selective for transuranian elements. Finally, to be REACH compliant, the method has been adapted to PS Sr-resin and validated for nuclear waste samples.
Experimental
Reagents and equipments
For selectivity tests, certified ICP standards including a mix of numerous elements (Ag, Al, As, B, Ba, Be, Ca, Cd, Co, Cr, Cu, Fe, K, Li, Mg, Mn, Mo, Na, Ni, Pb, Sb, Se, Sr, Si, Ti, Tl, V, Zn), are purchased from SPEX Certiprep or VWR or SCP Science (namely QC28, QC21, QC7 or single element standards). Those solutions are also used for the establishment of ICP-AES calibration curves. In this work, ICP-AES devices are implemented to characterize the selectivity, i.e. to determine which elements were present in the different fractions obtained at each step of the methods. Additionally, they are used to measure Sr recovery yields for each experiment. Two different instruments are available in the laboratory: a SpectroBlue® from Spectro Ametek and a PQ9000® from Analytik Jena. During a typical ICP batch sample run, blanks and quality control solutions (dilution of a QC28 standard) are added for quality control. The ISO 11352 standard is considered to estimate the ICP measurement uncertainties [26]. It is established at 5% for sample without digestion and 10% with digestion considering k = 1.
The Sr-resins® and TRU-resins® are purchased from Triskem. They are either conditioned in 2 mL column for gravitational flow separation or in 2 mL cartridge for separation on vacuum box. For some experiments, an automated separation system from ESI (prepFAST® station) was implemented and in-house cartridges with Sr-resin® were prepared. The Sr PS-resin were prepared at the University of Barcelona similarly to what was described in Ref. [23,24,25] and sent to our laboratory directly conditioned in 2 mL cartridges.
All the chemicals used in this experiment are of analytical grade and the water is of ultra-pure quality from a Milli-Q purification system supplied by Millipore (France). All the eluent solutions for separations are dilutions of concentrated nitric acid at 69% obtained from Merck or Supelco. For the Sr PS-resin, another eluent is needed [23,24,25]: a LiNO3 solution at a concentration of 6 M was prepared from a salt obtained from VWR. In the reference method based on Sr-resins®, other chemicals were used for precipitation steps: iron nitrate nonahydrate (Alfa Aesar) with an 18 g/L solution, concentrated ammonia at 28% (VWR) and saturated aqueous solution of Na2CO3 prepared from its anhydrous salt (Sigma Aldrich).
All the nuclear measurements (liquid scintillation and plastic scintillation) are done with the AccuFLEX LSC-8000® scintillation counter from Hitachi. The 20 mL polyethylene scintillation vials are purchased from Zinsser and the scintillation cocktails, Ultima Gold LLT®, from Perkin Elmer. The measurements last 10 min after 30 min waiting time. 90Sr is measured between 1 and 275 keV whereas 90Y is counted from 275.5 keV to 1500 keV. A blank is measured before each batch and removed from each sample measurement. For the preparation of spiked samples and calibration of the scintillation counter, a standard solution of 90Sr + 90Y is purchased from CERCA. The activity of the source was around 40 kBq/g. With our liquid scintillation counter, the detection efficiency was very close to 1 for 90Sr.
If necessary, the samples are digested with a microwave oven (Speed Wave from Berghof, Germany) with different acids depending on their nature.
Samples
As the goal of this paper is to validate a new protocol on radioactive waste samples, it is necessary to work on representative samples with a variety of different characteristics. Four samples which are the similar to the ones selected in a previous article [5] were chosen: a low activity level effluent (Sample 1), a contaminated effluent (Sample 2), spent ion exchange resins embedded in concrete (Sample 3) and a highly contaminated sludge (Sample 4). As the samples are received in the laboratory a long time after their conditioning, no 89Sr is detected because of its short period.
For sample 1, as it contained 90Sr below limit of detection (LOD), it was spiked with a known activity of 90Sr source. An ICP-AES measurement showed a low contamination of K and Na (respectively 1.1 mg/L and 1.5 mg/L at 10% k = 2) and traces of Ca, Si and Zn (approximately 0.1 mg/L) [5].
The samples 2, 3 and 4 had to be digested according to the in-house method presented in previous publications [5, 27].
Sample 2 contained 0.2 g/L of Na, 20 mg/L of Ca and 1 mg/L of Fe. About 5 mL of sample was digested with 10 mL of 69% nitric acid (HNO3). The main gamma emitter was Cs-137 with a mass activity of around 100 Bq/ml.
Sample 3 was digested with a mix of 10 mL of 69% HNO3 and 3 mL of 37% hydrochloric acid (HCl) with or without hydrofluoric acid (HF) depending on the experiments. Digested sample 3 contained a high content of Ca (around 0.7 g/L) because it is one of the main components of concretes with Si. Without HF in the digestion protocol, the Si is not completely digested. However, to avoid the hazardous manipulation of HF, it was first preferred to test the protocol without it initially. The main gamma emitters were Cs-137 and Co-60 respectively with mass activities of around 16,000 Bq/g and 80,000 Bq/g.
Sample 4 corresponds to an evaporator concentrate with similar activities as in Ref. [27]. This matrix is particularly complex due to high quantities of different cations and radionuclides. About 1 mL of sample was digested with 10 mL of 69% HNO3. The sample concentrations of Na, Ca and Fe were respectively around 70 g/L, 3 g/L and 1500 mg/L.
Reference method
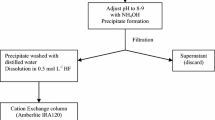
Many years ago, the in-house method based on Sr-resin® called here reference method has been developed for 90Sr determination in radioactive waste. In fact, the in-house procedure using successive precipitations with fuming nitric acid was found to be inaccurate for samples with high content of Ca, Ba or 89Sr [5]. The purification method begins with the addition of 2 mg of stable Sr to the sample solution (between 1 and 10 mL, depending on the sample, directly or in the digested solution). After an evaporation step, the residue is recovered in 8 M HNO3. Approximately 1 mg of Fe is added if the sample does not contain this Fe quantity. About 1 mL of concentrated ammonia is then added to the solution to induce the co-precipitation of lanthanides, actinides, and some metals at pH = 9–10. After a centrifugation step at 3500 rpm during 10 min, the precipitate is discarded and the supernatant is recovered. It is known that the supernatant obtained after ammonia precipitation contains Sr but also Ca, some metals such as Ni, Co if these latter are initially present in the sample [28]. Afterwards, a few milliliters of a saturated solution of Na2CO3 are added to the supernatant. The solution is heated on a hot plate until the appearance of a precipitate of SrCO3. At this point of the method, alkali metals and anionic compounds, except carbonates, remain in the supernatant [29]: they are removed by filtration on 0.45 µm syringe filters (Millex®). It must be noted that if Ca is present in the sample, it co-precipitates with SrCO3. 10 mL of 6 M nitric acid solution are then passed through the filter to dissolve the carbonate precipitate.
The resulting solution is then loaded on Sr-resin® (introduced in a column or a cartridge depending if a vacuum box is used or not) previously conditioned with 6 M nitric acid solution. At this step, Sr is retained on the resin. 20 mL of 4 M nitric acid solution is used to wash the resin and discard remaining interferents, such as Ca and Ba [13, 14]. Concerning Y, it was mainly precipitated at the precipitation step with ammonia but the possible remaining traces can also be eliminated during this washing step. Finally, Sr is eluted with 20 mL of 0.02 M nitric acid solution. After evaporation, the purified fraction is dissolved in 15 mL of 0.02 M nitric acid solution. This step allows to remove the possible remaining traces of volatile radionuclides such as 3H.
5 mL of the purified fractions are introduced in a liquid scintillation vial with 15 mL of scintillation cocktail. After shaking, the 90Sr activity is measured in the scintillation counter after 30 min waiting. 250 μL of the final solution are used for recovery determinations on stable Sr by ICP-AES. In this method, it can be noted that the stable Sr has two functions: carrier and tracer.
Developed method
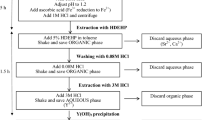
The new protocol was based on the same separation conditions for Sr-resin® as those described in the previous paragraph. It starts with the evaporation of the sample to be purified into dryness. The residue was recovered in 10 mL of 6 M nitric acid solution. The obtained solution is directly loaded on a TRU-column® previously conditioned with 6 M nitric acid solution. After passing through the resin, it is collected at the end of the column: the fraction contains Sr which is not retained in those conditions on TRU-resin®. The collected solution is directly loaded on Sr-column®, also conditioned with 6 M nitric acid. If the separation is carried out with cartridges with a vacuum box, the top of Sr-cartridge® is placed and connected to the bottom end of TRU-cartridge®. Once the solution has passed through the two cartridges, the TRU-cartridge® is removed. Then, the protocol includes the same steps as described before: a washing with 20 mL of 4 M nitric acid solution and an elution but with only 15 mL of 0.02 M nitric acid instead of 20 mL. As the evaporation step has already discarded volatile radionuclides, the evaporation is not repeated at the end of the procedure. Moreover, it was checked that a volume of 15 mL is large enough to obtain the same Sr recovery yields. Both modifications are time saving. At the end, the 90Sr activity is measured according to the same procedure as previously described and the recovery yield is determined by ICP-AES. A schematic of the developed method is presented in Fig. 1.
Method adapted to Sr PS-resin
To eliminate the use of scintillation cocktails and obtain a REACH compliant protocol, the optimized method previously presented was adapted to the plastic scintillation resin called here Sr PS-resin. As recommended in previous publications [25], 5 mg of stable Sr are used as tracer for yield determination. At first, after addition of stable Sr, the sample is evaporated to dryness and dissolved with 6 M HNO3. The solution is transferred to the reservoir placed on the top of the two cartridges containing the selected resins (TRU-resin® and Sr PS-resin) for the implementation of the vacuum box. Indeed, for the moment, the Sr PS-resins are conditioned in cartridges. Prior to the sample loading, the resins were conditioned with 6 M HNO3.
Before operating the vacuum pump, a tared vial must be positioned in the vacuum box in order to recover all the loading and washing fractions. Then, the vacuum pump can be started and the solution passes through both cartridges. The elution rate must be approximately one drop per second. After the solution has completely passed through the resins, the TRU-resin® cartridge is removed and a new reservoir is placed on the Sr PS-resin cartridge. Then, 15 mL of 4 M HNO3 are used for a first washing of the resin. A second washing is performed with 4 mL of 6 M LiNO3 aqueous solution. This step eliminates nitric acid, limits chemiluminescence and maximizes counting efficiency during nuclear measurements [23]. When washings are finished, the vacuum is pushed to 0.6 Bar during at least 10 min to dry the Sr PS-resin. Once dried, the cartridge is removed from the vacuum box and placed directly in the scintillation vial. The geometry of the cartridge has been adapted to standard scintillation vial but it must be positioned vertically and upright in the vial. Finally, the scintillation vial is placed in the scintillation counter for the measurement after 30 min waiting.
The tared vial used to recover all the fractions is then replaced in the vacuum box to collect the washing fraction of the TRU-resin® with 5 mL of water. The final solution is weighed, homogenized and a known volume is taken to perform the dilution prior to ICP-AES analysis. It will be used to determine the overall Sr yield of the method. In the case of Sr PS-resin, the recovery yield is calculated indirectly by determining the Sr quantity collected in the vial.
The last step is to verify the separation by measuring the 90Y ingrowth the days following the separation. The complete process on Sr PS-resin is illustrated in Fig. 2.
Results and discussion
Reference method
The reference method of the laboratory provides accurate 90Sr results [30] but can present low Sr recoveries. Due to the complexity of the samples analysed in the laboratory, it is difficult to identify which analyte or matrix is responsible for this loss. For example, values of Sr yields smaller than 10% were observed for clear effluents with low level of radioactivity and also for more complex samples, such as effluents with a moderated content of suspended solids and concretes. Different assumptions have been put forward based on experimental observations and theoretical knowledge of the method. The first hypothesis was that the presence of F− can induce weak Sr yields. Indeed, Sr is known to precipitate easily with F− as SrF2 (solubility product value Ksp of around 5 × 10−9 and a solubility of 0.1 g/L in water) [31]. The formation of SrF2 precipitate can explain the unsatisfactory results obtained for concretes digested with a mixing of HNO3 and HF but not for simple effluents where no F− was detected in solution. Thus, a more detailed study was undertaken by taking into consideration the composition of the samples. After the digestion step, common elements were found in significant quantity (> 1 mg in the analysed aliquot) in “problematic” samples: Al, Ca, Fe, Si, NO3−, Cl− and F−. The presence of anions was mainly related to acids used for digestion: HNO3, HCl and HF knowing that HF is only added for concretes in the laboratory. First, a focus was placed on the evaluation of the Sr loss during precipitation steps with ammonia and carbonate. Synthetic solutions were prepared with the elements previously identified. Other analytes which are known to have a similar chemical behaviour to Sr or to be present in significant content were added complementarily. As Pb is highly retained on Sr-resin® [12,13,14], it was interesting to determine its behaviour in the precipitation steps. As reminder, stable Fe was systematically added for ammonia precipitation in the reference protocol. Sr was measured at each step of the pretreatment in the presence of ten analytes: alkali metal (Li), alkali-earth metals (Ca, Ba), transition metals (Ag, Al, Co, Mn, Pb) and metalloid (Si). Their cumulative recovery yields are given in Fig. 3. It can noted that the composition of the carbonate precipitate was determined after its dissolution in 6 M nitric acid which corresponds to “Carbonate precipitate dissolved before Sr-resin” in Fig. 3.
As expected, Ba and Ca behaved similarly to Sr and were not separated during the pretreatment with precipitations, which is not problematic since the selectivity of the Sr-resin® is supposed to solve the problem [14]. However, it is necessary to be careful with the quantity of Ca present in order to avoid a retention capacity loss of the Sr-resin®. From the literature [14], it was highlighted that the capacity can be affected above a concentration of ~ 10−2 M. Al, Mn and Pb were discarded during ammonia coprecipitation with Fe but not Ag and Co. The latter is known to form various complexes with NH3 which prevents its precipitation in basic medium [28]. It is important to note that ammonia precipitation is also useful to discard actinides [29] such as 241Pu which is a problematic interferent of 90Sr measurement by liquid scintillation. Ag and Co were eliminated in the next stage with carbonate precipitation. Alkali metals (K, Li) behaved similarly to Ag and Co: they did not precipitate in ammonia medium but they were removed with carbonate precipitation. Si was not detected in the solution recovered in 8 M HNO3 after dry evaporation. A cloudy solution was observed after addition of 8 M HNO3 that could be attributed to insoluble Si. The obtained cloudy solution was filtered on Millex® filter before ICP-AES analysis, otherwise the nebulizer could be clogged with the suspended solids. This step explained the almost complete absence of Si and it can be deduced that the loss of Si was not corroborated to the one of Sr.
The overall Sr recovery yield after the pretreatment was around 65%, which is satisfying for a complete purification process but not for only the first steps of a method applied to a simple synthetic solution, as it is the case here. No important loss of Sr was noticed after evaporation and carbonate precipitation. In contrast, a significant loss of around 30% was observed during ammonia precipitation. This result was in agreement with a previous work which showed a 30% loss in presence of Fe, Ni, Co, Ba, Eu and Cs [32]. Another test was performed with a multi-element solution (named QC28 from VWR): in this case, the loss of Sr was lower but was still significant. Consequently, the loss of Sr during our reference method can be variable depending on the sample composition but it originated from ammonia precipitation, maybe by adsorption of Sr on iron hydroxides [33]. Consequently, it was decided to discard this step and to investigate a new pretreatment which would be robust for all compositions of samples.
For that purpose, the selectivity of Sr-resin® was studied in our chromatographic conditions to better guide our choice towards the new pretreatment step. Horwitz et al. [14] only reported the behaviour of common elements and fission products in 3 M HNO3 + 0.01 M oxalic acid. Experiments on Sr-resin® were carried out with a multi-element QC28 solution (1 mg of each element in the sample solution). As expected, Sr-resin® has a very high selectivity towards alkali metals, alkali-earth metals and transition metals. 96% of stable Sr was recovered in 0.02 M HNO3 and only Ba was detected in the Sr fraction (around 8% of the initial quantity). From Ref. [14], it is known that Ba has a behaviour close to Sr on Sr-resin®, which can explain the presence of Ba in the final fraction. The remaining Ba might only be disturbing in case of radioactive barite concretes but these samples are rarely encountered in the laboratory. If such samples had to be analysed, gamma spectrometry analysis should be carried out to check whether the Ba-133 decontamination is satisfactory or not. If Ba-133 elimination is not sufficient, a second separation on Sr-resin® can be implemented to complete its removal. Tetravalent actinides which were not considered up to now are supposed to behave similarly to Sr [14], which can be problematic for samples containing 241Pu, a beta emitter interfering with 90Sr. The addition of oxalic acid as a competitive complexing agent in the eluent was demonstrated to induce the elimination of Ba and actinide elements [14] while Sr was still retained on Sr-resin®. Based on the high selectivity of Sr-resin®, it was decided to perform a single purification step on Sr-resin® with and without oxalic acid. The procedure without pretreatment before Sr-resin® was applied to three types of radwaste samples: a low contaminated effluent spiked with 90Sr and two more complex samples containing Pu and 90Sr notably. As some samples were contaminated with Pu, the influence of oxalic acid was evaluated: oxalic acid at a concentration of 0.05 M was added or not in 4 M HNO3 washing solution. The results were compared to our reference value and the normalized errors (En) were calculated for the three real samples (see Table 1) according to the formula given in Ref. [28]. A En value lower than 1 means that there is no significant difference between the reference method and the developed method without pretreatment.
As expected, the method based on a single purification on Sr-resin® was only accurate for low contaminated effluent. A En value of 0.7 allowed to validate the result with a Sr yield of 93 ± 10%. For this sample, since no bias was detected without pretreatment, the washing with oxalic acid was not tested. As a result, a fast and simple method is available for samples with low contamination but is not suitable for all natures of nuclear waste samples. Indeed, for samples 2 and 3, when only implementing Sr-resin®, the measured 90Sr activities were significantly higher in comparison to the reference values whereas the Sr yields were higher than 90%. This overestimation can be linked to the presence of interferents, such as 241Pu in the purified Sr fraction. For samples 2 and 3, oxalic acid was added in the washing solution but it had not the expected efficiency. A decrease of activity was observed for both samples (− 9% for sample 2 and − 7% for sample 3 respectively) compared to the method without oxalic acid, which can correspond to a partial elimination of interferents. However, the En values remained significantly higher than 1. A low efficiency of oxalic acid was already highlighted in a previous publication from Grate et al. [34] who noticed that only 10% of Pu(IV) was discarded with oxalic acid. A complementary test was undertaken with an oxalic acid concentration 10 times higher than previously but no significant improvement was observed. In conclusion, a purification method based on Sr-resin® with the addition of oxalic acid in the washing solution is not adapted to provide reliable 90Sr results for various types of radwaste samples. Therefore, it is necessary to find a new robust pretreatment to eliminate interferents, especially tetravalent actinides, prior to the separation on Sr-resin®.
Developed method
In order to solve the problems described previously, it was decided to implement a TRU-resin® prior to Sr-resin® to discard actinides. This approach has already been proposed for 90Sr analysis in urines [35], environmental soils [36, 37] or radioactive wastes [38] with precipitation steps at the start of the protocol. The TRU-resin® is known for its high selectivity towards actinides and does not retain Sr when using nitric acid eluent with a concentration between 1 and 8 M [36, 37]. As a preliminary study, a synthetic sample with Sr and 27 stable analytes was purified with a protocol based on TRU-resin® and Sr-resin®. The distributions of all elements in the different fractions of the protocol were determined in order to precisely characterize the selectivity of the developed method (see Fig. 4). To determine which elements were retained on the TRU-resin® with 6 M HNO3, the resin was rinsed with water after the separation: this fraction is named “retained on TRU-resin®” in Fig. 4.
Figure 4 shows that, in the final fraction (named Sr elution), only Sr was detected with a very high recovery yield of around 97%. This value is comparable to the one obtained with the Sr-resin® method without pretreatment (> 90%) which confirms the fact that Sr was not fixed at all on TRU-resin® when using 6 M HNO3. Moreover, it can be seen that Ba was not detected in the Sr fraction whereas traces of Ba were observed with the method without pretreatment. The separation without pretreatment was realized with an automated separation system and the optimized method with TRU-resin® with a vacuum box. The presence of Ba in the elution fraction in the first experiments could be due to the speed of elution or some other parameters that should be optimized. The washing of the TRU-resin® with water shows that Fe, Mo, and Ti were retained in our chromatographic conditions. Their recovery was not quantitative when using water as rinsing solution because they were highly fixed and water has not a sufficient elution capacity, confirming previous publications [38,39,40]. Ag was not observed whatever the fractions analysed. Since the previous experiments showed that Ag was not retained on Sr-resin®, it can be deduced that it was fixed on TRU-resin®. As expected, the other analytes were discarded during the loading on TRU-resin®. Concerning Pb, it was not detected in any fractions because it was highly retained on Sr-resin® [14] and not eluted in our conditions. Si was not recovered quantitatively due to the same problem of solubility as mentioned above.
Therefore, this first investigation performed on non-radioactive elements demonstrated the potentiality of the developed procedure based on TRU-resin® and Sr-resin®: a purified fraction of Sr was obtained with a high recovery yield. The next step of the work was to verify the elimination of actinides and particularly Pu. For that purpose, the new method was applied for 90Sr analysis in the same types of radioactive samples as previously tested: contaminated effluent, concrete, and sludge. The results in terms of En values are presented in Table 2. For the investigations reported in Table 2, the concrete was digested with a mixing of HNO3 and HCl.
Comparing to reference activities, the results on samples 2 and 3’ can be validated with En values of respectively 0.1 and 0.02. The Sr yield values were respectively 89 ± 10% for sample 2 and 89 ± 10% for sample 3. It is only 4% below the Sr yield obtained without pretreatment for sample 1. It means that the loss of Sr due to the TRU-resin® is minimal even with complex samples. As a reminder, the method without pretreatment did not work for these two samples. Comparing to the reference method, an additional advantage is the decrease of the number of steps: two precipitations steps implying a centrifugation and a filtration become one separation step on two extraction resins. Moreover, in the perspective of a potential automation, the separation on resins can be adapted more easily than precipitations.
Recently, publications investigated the use of 90Y ingrowth to perform a rapid 90Sr measurement without waiting for secular equilibrium [41,42,43]: accurate 90Sr results were obtained within a few days. This approach enables to gain time without reducing analysis quality. It can also be applied to control the selectivity of 90Sr purification and the elimination of interfering radionuclides, which is of prime interest for complex samples such as radioactive waste. Indeed, the ingrowth of 90Y will be biased by the presence of interferents [44]. In the present work, it was decided to compare the experimental ingrowth factor related to 90Y (noted IF) with the theoretical value calculated from the following equation.
where \({A}_{tot}\) is the total activity for any time t, \({A}_{tot}^{0}\) is the total activity for zero time, \({lambda}_{Sr}\) and \({lambda}_{Y}\) are respectively the decay constants of 90Sr and 90Y.
Equation 1 was derived from Bateman’s work [43, 44]: it was simplified since the period of 90Sr is largely higher than the one of 90Y. The theoretical IF values were calculated at 1.23, 1.84 and 1.97 respectively after 1, 7 and 14 days of ingrowth, in agreement with the data given in Ref. [43]. If an interfering radionuclide, for example 241Pu, is still present in the purified fraction after the separation, the IF value will be too low in comparison to the theoretical value.
Before applying this approach to a real sample, a 90Sr source with a known activity was purified on Sr-resin® and the purified fraction was measured just after the separation, then 1 day, 7 days and 14 days after the separation (noted respectively as T + 1 day, T + 7 days, T + 14 days). The experimental IF values were calculated as the ratio of the total counts of the whole spectra at a given time over the initial total counts. Values of 1.26, 1.82 and 1.95 (with an uncertainty of 1%) were determined respectively at T + 1 day, T + 7 days, T + 14 days. The differences between the experimental and theoretical IF values were lower 3%, which validates the approach. Consequently, the IF was checked after several days following the separation process for real samples. For samples 2 and 3’, the IF values measured at T + 14 days were respectively 1.98 (± 1%) and 1.86 (± 1%), which corresponds to a difference of 2% and 6% in comparison to the theoretical value of 1.97. As the difference was below 10%, it can be deduced that no interferent was present in the purified fractions and that the measured 90Sr activity is accurate for samples 2 and 3 (contaminated effluent and spent ion exchange resin embedded in concrete).
For sample 4 (sludge), the Sr yield was still very satisfactory (96% ± 10%) but a En value of 2 was determined, which is too high to validate the result: the 90Sr activity is overestimated. As for the other samples, the approach of 90Y ingrowth was applied: an IF value of 1.61 (± 3%) was found at T + 7 days, meaning a difference of 13% below the expected value of 1.84. The incorrect values of En and IF indicate that a β emitter interferent was not efficiently discarded during the process and biased the measurement. The sludge sample was similar to the one studied in Ref. [27]: it is known that it contains many different radionuclides including Pu in significant quantity (measurements of 239/240Pu and 241Am + 238Pu by alpha spectrometry and 241Pu by liquid scintillation after separation) and a high salt content. The most likely assumption is that the elimination of 241Pu might be not complete with this complex sample. In fact, from our experimental results, it can be inferred that the interfering radionuclide is not sufficiently retained on the TRU-resin® and it behaves in a similar way to Sr on the Sr-resin®. From literature [13, 40], it can be supposed that the most probable radionuclide is one beta isotope of Pu so 241Pu.
To solve this problem, an experiment was carried out with two TRU-resin® columns instead of one. Indeed, for sample 4, the capacity of the TRU-resin® could have been exceeded or interferents in high quantity (ex: U) or a high salt content could have reduced the capacity of the TRU-resin® as it was observed with Fe on TRU-resin® [40]. Sample 4 has a high content of metals in a matrix of 2 M NaNO3, which can lower the efficiency of TRU-resin®. For both cases, the Pu might be not retained completely on TRU-resin® and in consequence, eluted with Sr in the final fraction. For the protocol based on two TRU-resin® columns, the volume of sample solution loaded on the column was still 10 mL. The only change was the addition of a second TRU-resin® column between the first one and the Sr-resin® column. The results obtained for sample 4 are given in Table 3.
With the new procedure, the En value was found at 0.05 and the IF value was determined at 1.79 at T + 7 days, meaning a difference of only 3% with the theoretical value. Consequently, the addition of a second TRU-resin® is an efficient solution for the purification of 90Sr in highly contaminated samples such as sludges. The probability than the issue comes from 241Pu is high because it is also a pure beta emitter and a second TRU-resin® solved the problem. However, there is no definitive proof from this experiment. Another important point to underline is that the Sr yield was not affected by the second TRU-resin® with a value of 92 ± 10%. In conclusion, to analyze complex samples with high Pu content, two columns (or cartridges) of TRU-resin® are necessary to provide accurate 90Sr measurements.
As a reminder, one of the main issues related to our reference method based on precipitations and Sr-resin® was the drastic loss of Sr in case of concretes digested with HF. The developed method was applied to such samples: it provided a very satisfactory recovery yield of 95% and the bias compared to the reference method was lower than 10%, which enables to achieve our initial objective of high Sr yields whatever the studied matrix. Finally, the method was evaluated during a European NKS intercomparison [30]: the z-score obtained was satisfactory (see lab 7), which clearly proves the accuracy and robustness of the developed method.
Method adapted to Sr PS-resin
As the developed method provided satisfying 90Sr results for various types of radioactive waste, it was decided to investigate the potentialities of the Sr PS-resin. The aim is to eliminate the use of scintillation cocktails uncompliant with REACH regulation while preserving the performances of the purification method.
With the scintillation counter available in the laboratory, the liquid scintillation measurement is based on the determination of a detection efficiency at each measurement from a quenching curve previously established with standard solutions [45]. For the plastic scintillation measurement, another strategy was applied as it has already been done in previous publications [23,24,25]. An average detection efficiency was determined by repeating separations with known activities of 90Sr source and then applied on every sample. Sáez-Muñoz et al. [25] demonstrated that the amount of Sr fixed on the resin had a great influence towards the detection efficiency: the greater the Sr mass is, the lower the efficiency is. Consequently, during the different experiments, various stable Sr masses were added to take into account this factor. The eleven values of detection efficiency obtained are illustrated in Fig. 5 and compared with the data reported by Sáez-Muñoz et al. [25].
Detection efficiency for 90Sr measurement on Sr PS-resin as a function of stable Sr mass retained on Sr PS-resin (red). Experimental data of Sáez-Muñoz et al. [25] were added for comparison (black). (Color figure online)
Our experimental points did not show a clear tendency for the range of Sr masses investigated. Comparing these results with the ones obtained by Sáez-Muñoz et al. [25], it can be seen that the two studies were in agreement as the values reported by Sáez-Muñoz et al. are included in the scattering of our points, except for the data related to 5 mg. The difference related to the 5 mg mass can be explained by the differences of scintillation counters. For the range of investigated Sr masses, between 2.7 mg and 4.2 mg of Sr fixed on the Sr PS-resin, the detection efficiency can be considered as a constant: the average detection efficiency obtained was 0.91 ± 10% for our studies. From these experiments, it can be deduced that the Sr yield should be between 50 and 100% to consider the impact on detection efficiency as unsignificant. As reminder, the detection efficiency was very close to 1 for 90Sr measured in liquid form with our scintillation counter. The detection efficiency obtained with the Sr PS-resin is high even if it is slightly below compared to liquid scintillation, which is very promising for 90Sr determination in radioactive waste.
Once the average detection efficiency has been assessed for our procedure, two aliquots of a 90Sr source solution were purified respectively on Sr-resin® for liquid scintillation measurement and on Sr PS-resin for plastic scintillation measurement. The Sr yields obtained were determined at 86% for Sr-resin® and 80% for Sr PS-resin respectively: the values are very close in agreement with Ref. [23,24,25]. The spectra obtained with our scintillation counter are presented in Fig. 6 for both separations just after the separation and 10 days after the separation. The spectra were normalized with these recoveries to be compared directly.
The 90Sr spectra obtained at T = 0 had a very similar pattern, which is in agreement with our previous experiments since the detection efficiencies were very close. However, at T + 10 days, the spectra were found to be slightly different. With plastic scintillation, the overlap between 90Sr and 90Y was more important: 90Y signal was more quenched and was shifted to lower energy, which could have been problematic if one had tried to measure 90Y alone.
A synthetic sample with a multi-elemental QC28 solution (1 mg of each interferent in the sample solution and 5 mg of Sr) and a known activity of 90Sr from a source was purified with the protocol adapted for Sr PS-resin including the pretreatment with TRU-resin®. The distribution of the 27 interferents and Sr are presented in Fig. 7 at each step of the procedure. The differences between the two methods were the use of the 6 M LiNO3 aqueous solution for the second washing step (it allowed to replace nitric acid that would create chemiluminescence during LSC measurement [23,24,25]) and the removal of the Sr elution step.
As expected, the separation was similar than the one observed for Sr-resin® with TRU-resin® as pretreatment (see Fig. 5). Indeed, the extractant impregnated is the same for Sr-resin® and Sr PS-resin, so the selectivity and the Sr recovery yield must be very close. For this experiment, the Sr recovery yield reached 84%, which proves the efficiency of the separation. The presence of a great number of analytes did not affect the performance of the Sr PS-resin.
Similarly to Sr, it was determined by indirect measurement that Pb was fixed on the Sr PS-resin, which can bias the detection efficiency applied for every sample. Fortunately, stable Pb was not present in significant quantity in the usual samples encountered at the laboratory. However, the influence of Pb was checked: in the present experiment, 90Sr was measured in the presence of 1 mg of Pb. The value of En obtained (calculated between the reference value of the source and the measurement performed by plastic scintillation after separation) is 0.2, meaning that stable Pb, up to 1 mg, had no significant influence on 90Sr measurement. An initial ICP-AES measurement could ensure that the amount of Pb is not too high in case of particular samples. The potential presence of 210Pb (a beta emitter formed from the disintegration chain of 238U which disintegrates into 210Bi, which is also a beta emitter [46]) could induce a bias during 90Sr measurement. However, as the couple 210Pb/210Bi has different half-lives (respectively 22.23 years and 5.012 days [46]) in comparison to the couple 90Sr/90Y, the presence of those radionuclides would be detected during the measurement of 90Y ingrowth which would be biased.
To verify the quality of 90Sr separation, the measurement of 90Y was performed similarly to the previous method on Sr-resin®. It must be noted that a special care must be paid to the drying stage of the Sr PS-resin. An incomplete drying of the resin did not affect 90Sr result but the 90Y ingrowth was lower than expected. After a drying of at least 10 min with a vacuum of 0.6 bar, the problem was solved. Measurements of 90Y ingrowth were performed from a 90Sr source after several days of separation on Sr PS-resin. The experimental IF results are compared to the theoretical curve of 90Y ingrowth in Fig. 8. As reminder, they corresponds to the total counts of the whole spectra at a given time divided by initial total counts.
Even though the signals of 90Sr and 90Y are overlapped with the Sr PS-resin (see Fig. 6), it does not affect the establishment of the experimental IF curve. Moreover, the experimental IF values fit quite perfectly with the theoretical curve. Therefore, it is valid to use 90Y ingrowth to check the efficiency of the separation and the elimination of all interferents (such as 241Pu and 210Pb) for the method adapted to Sr PS-resin.
The validation of the method based on Sr PS-resin was demonstrated by the analysis of real samples. As previously, three types of radioactive waste were considered: a low contaminated effluent (sample 1), an ion exchange resin embedded in concrete (sample 3) and a sludge (sample 4). For the effluent, only one TRU-resin® column was implemented before Sr PS-resin. For samples 3 and 4 that are more complex, the use of two TRU-resin® columns prior to Sr PS-resin was preferred as pretreatment. The results are presented in Table 4.
The results obtained showed the efficiency of the method with En values of 0.1 for sample 1, 0.7 for samples 3 and 4, respectively. Moreover, the separations in term of selectivity were validated with the measurements of 90Y ingrowth after 7 days for sample 1 and 3 and 9 days for sample 4, respectively. With a bias of only 2% with the expected ratios, the absence of remaining interferents was confirmed for the three samples. Despite the complexity of samples 3 and 4 (concrete and sludge), the method adapted to Sr PS-resin associated with one or two TRU-resins® as pretreatment was validated on real radwaste samples. This fast protocol (only half day) provided the elimination of the elution step and thus the simplification of the method. Moreover, the greatest advantage was the removal of scintillation cocktails for the 90Sr nuclear measurement. The protocol is thus compliant with the European REACH regulation. First and foremost, it is an important progress for laboratory technicians who can avoid the use of dangerous products and also for environmental issues.
Conclusion
According to the French regulation, it is necessary to quantify 90Sr in nuclear waste. As a pure β emitter, it must be separated from its matrix and interferents with a radiochemical method prior to its measurement. Since its development, the chromatographic extraction Sr-resin® is the separation material preferred by the analytical laboratories due to its efficiency for a wide range of samples. With our in-house reference method based on ammonia and oxalate precipitations followed by a separation on Sr-resin®, problems of low Sr recovery yields were encountered. The problematic step was identified as the precipitation step in ammonia where Sr co-precipitates with Fe even in simple samples. Before investigating a new procedure, it was checked whether a single separation with Sr-resin® resin was sufficiently selective, which is not the case for wastes with medium and high radioactivity level. In consequence, an alternative pretreatment without precipitation steps was developed with TRU-resin®. This choice was made on the basis of the recognized capabilities of this resin to eliminate actinides specifically. The efficiency of this new method was tested successfully on representative nuclear waste samples: effluent, concrete, and sludge. For complex samples with especially high content of 241Pu, it was demonstrated that two successive TRU-resins® are necessary to obtain accurate 90Sr analysis. The determination of 90Y ingrowth enabled to check the selectivity of the developed procedure. Despite the efficiency of the new optimized method, it is not REACH compliant due to the use of scintillation cocktails for 90Sr nuclear measurement by liquid scintillation.
The developed method was adapted to the use of a plastic scintillation resin selective towards Sr namely Sr PS-resin that enables nuclear measurement by plastic scintillation without the use of scintillation cocktails. After the study of the selectivity and the efficiency of the method based on TRU-resin® and Sr PS-resin, it was validated on three radwaste samples including complex matrices: concrete and sludge. The advantages (no scintillation cocktail, simplification of the manipulation) and the performances in term of selectivity and counting efficiency make the Sr-PS resin an advantageous choice for the characterization of 90Sr in nuclear waste. The possibility to extend the implementation of plastic scintillation for other radionuclides offers a promising perspective.
References
International Atomic Energy Agency (2020) PRIS-Power Reactor Information System. https://pris.iaea.org/PRIS/home.aspx. Accessed 31 Oct 2023
LNE-LNHB/CEA (2005) Nuclear data-90Sr. http://www.lnhb.fr/nuclides/Sr-90_tables.pdf. Accessed 31 Oct 2023
Willard HH, Goodspeed EW (1936) Separation of strontium, barium, and lead from calcium and other metals-by precipitation as nitrates. Ind Eng Chem Anal Ed 8:414–418. https://doi.org/10.1021/ac50104a003
Sunderman DN, Meinke WW (1957) Evaluation of radiochemical separation procedures. Anal Chem 29:1578–1589. https://doi.org/10.1021/ac60131a005
Baudat E, Gautier C, Fichet P et al (2021) Optimization of Sr-90 precipitation in nitric acid using design of experiments for radioactive waste characterization method. J Radioanal Nucl Chem 328:637–650. https://doi.org/10.1007/s10967-021-07680-5
Shao Y, Yang G, Tazoe H et al (2018) A review of measurement methodologies and their applications to environmental 90Sr. J Environ Radioact 192:321–333. https://doi.org/10.1016/j.jenvrad.2018.07.013
Cobb J, Warwick P, Carpenter RC, Morrison RT (1994) Determination of strontium-90 in water and urine samples using ion chromatography. Analyst 119:1759–1764. https://doi.org/10.1039/AN9941901759
Petrow HG (1965) Rapid determination of strontium-90 in bone via solvent extraction of yttrium-90. Anal Chem 37:584–586. https://doi.org/10.1021/ac60223a037
Baratta EJ, Reavey TC (1969) Rapid determination of strontium-90 in tissue, food, biota, and other environmental media by tributyl phosphate. J Agric Food Chem 17:1337–1339. https://doi.org/10.1021/jf60166a012
Suzuki Y, Ohara R, Matsunaga K (2017) Optimization of collision/reaction gases for determination of 90Sr in atmospheric particulate matter by inductively coupled plasma tandem mass spectrometry after direct introduction of air via a gas-exchange device. Spectrochim Acta Part B At Spectrosc 135:82–90. https://doi.org/10.1016/j.sab.2017.07.007
Russell B, García-Miranda M, Ivanov P (2017) Development of an optimised method for analysis of 90Sr in decommissioning wastes by triple quadrupole inductively coupled plasma mass spectrometry. Appl Radiat Isot 126:35–39. https://doi.org/10.1016/j.apradiso.2017.01.025
Horwitz EP, Dietz ML, Fisher DE (1991) Separation and preconcentration of strontium from biological, environmental, and nuclear waste samples by extraction chromatography using a crown ether. Anal Chem 63:522–525. https://doi.org/10.1021/ac00005a027
Horwitz EP, Chiarizia R, Dietz ML (1992) A novel strontium-selective extraction chromatographic resin. Solvent Extr Ion Exch 10:313–336. https://doi.org/10.1080/07366299208918107
Horwitz EP, Dietz ML, Chiarizia R (1992) The application of novel extraction chromatographic materials to the characterization of radioactive waste solutions. J Radioanal Nucl Chem 161:575–583. https://doi.org/10.1007/BF02040504
AFNOR (2002) Standard NF M60-316-Nuclear energy-Nuclear fuel technology-Waste-Strontium 90 assay in liquid or solid waste after a preliminary chemical separation
Eichrom Technologies, LLC (2014) Strontium-89/90 in water
Zhou Z, Ren H, Zhou L, Wang P, Lou XM, Zou H, Cao YY (2023) Recent development on determination of low-level Sr-90 in environmental and biological samples: a review. Molecules 28:90–110. https://doi.org/10.3390/molecules28010090
ECHA-REACH Substance Infocard-4-Nonylphenol, branched and linear, ethoxylated. https://echa.europa.eu/fr/substance-information/-/substanceinfo/100.239.148. Accessed 31 Oct 2023
Vasile M, Loots H, Vercammen L et al (2022) A study for the selection of NPE-free cocktails for LSC routine measurements. J Radioanal Nucl Chem 331:1–9. https://doi.org/10.1007/s10967-022-08405-y
Varlam C, Vagner I, Faurescu D (2019) Performance of nonylphenol-ethoxylates-free liquid scintillation cocktail for tritium determination in aqueous samples. J Radioanal Nucl Chem 322:585–595. https://doi.org/10.1007/s10967-022-08405-y
Tarancón A, Alonso E, Garcı́a JF, Rauret G (2002) Comparative study of quenching correction procedures for 90Sr/90Y determination by Cerenkov, liquid scintillation and plastic scintillation techniques. Anal Chim Acta 471:135–143. https://doi.org/10.1016/S0003-2670(02)00710-9
Tarancón A, Garcı́a JF, Rauret G (2004) Determination of beta emitters (90Sr, 14C and 3H) in routine measurements using plastic scintillation beads. Nucl Instrum Methods Phys Res Sect Accel Spectrometers Detect Assoc Equip 516:602–609. https://doi.org/10.1016/j.nima.2003.08.172
Bagán H, Tarancón A, Rauret G, García JF (2011) Radiostrontium separation and measurement in a single step using plastic scintillators plus selective extractants. Application to aqueous sample analysis. Anal Chim Acta 686:50–56. https://doi.org/10.1016/j.aca.2010.11.048
Sáez-Muñoz M, Bagán H, Tarancón A et al (2019) Rapid methods for radiostrontium determination in aerosol filters and vegetation in emergency situations using PS resin. J Radioanal Nucl Chem 322:1397–1408. https://doi.org/10.1007/s10967-019-06779-0
Sáez-Muñoz M, Bagán H, Tarancón A et al (2018) Rapid method for radiostrontium determination in milk in emergency situations using PS resin. J Radioanal Nucl Chem 315:543–555. https://doi.org/10.1007/s10967-017-5682-3
(2013) ISO 11352-Water quality-Estimation of measurement uncertainty based on validation and quality control data
Gautier C, Laporte E, Lambrot G, Giuliani M, Colin C, Bubendorff J, Crozet M, Mougel C (2020) Accurate measurement of 55Fe in radioactive waste. J Radioanal Nucl Chem 326:591–601. https://doi.org/10.1007/s10967-020-07332-0
Gautier C, Colin C, Garcia C (2016) A comparative study using liquid scintillation counting to determine 63Ni in low and intermediate level radioactive waste. J Radioanal Nucl Chem 308:261–270. https://doi.org/10.1007/s10967-015-4301-4
Lehto J, Hou X (2011) Chemistry and analysis of radionuclides: laboratory techniques and methodology. Wiley, Hoboken
Leskinen A, Dorval E, Baudat E, Gautier C, Stordal S, Salminen-Paatero S (2023) Intercomparison exercise on difficult to measure radionuclides in spent ion exchange resin. J Radioanal Nucl Chem 332:77–94. https://doi.org/10.1007/s10967-022-08687-2
Fullam HT (1976) The solubility and dissolution behavior of 90SrF2 in aqueous media. BNWL-2101, Battelle, Pacific Northwest Laboratories
Hou X, Østergaard LF, Nielsen SP (2005) Determination of 63Ni and 55Fe in nuclear waste samples using radiochemical separation and liquid scintillation counting. Anal Chim Acta 535:297–307. https://doi.org/10.1016/j.aca.2004.12.022
Benjamin MM, Leckie JO (1981) Multiple-site adsorption of Cd, Cu, Zn, and Pb on amorphous iron oxyhydroxide. J Colloid Interface Sci 79:209–221
Grate JW, Egorov O, Fadeff SK (1999) Separation-optimized sequential injection method for rapid automated analytical separation of 90Sr in nuclear waste. Analyst 124:203–210. https://doi.org/10.1039/A807468B
Tomita J, Takeuchi E (2019) Rapid analytical method of 90Sr in urine sample: Rapid separation of Sr by phosphate co-precipitation and extraction chromatography, followed by determination by triple quadrupole inductively coupled plasma mass spectrometry (ICP-MS/MS). Appl Radiat Isot 150:103–109. https://doi.org/10.1016/j.apradiso.2019.05.026
Qiao J, Salminen-Paatero S, Rondahl SH et al (2017) Inter-laboratory exercise with an aim to compare methods for 90Sr and 239,240Pu determination in environmental soil samples. J Radioanal Nucl Chem 314:813–826. https://doi.org/10.1007/s10967-017-5385-9
Sahli H, Röllin S, Putyrskaya V et al (2017) A procedure for the sequential determination of radionuclides in soil and sediment samples. J Radioanal Nucl Chem 314:2209–2218. https://doi.org/10.1007/s10967-017-5621-3
Grahek Ž, Rožmarić Mačefat M (2004) Isolation of iron and strontium from liquid samples and determination of 55Fe and 89, 90Sr in liquid radioactive waste. Anal Chim Acta 511:339–348
Quidelleur S, Granet M, Laszak I et al (2009) One step U-Pu-Cs-Ln-steel separation using TRU preconditioned extraction resins from Eichrom for application on transmutation targets. J Radioanal Nucl Chem 280:507–517. https://doi.org/10.1007/s10967-009-7449-y
Horwitz EP, Chiarizia R, Dietz ML, Diamond H, Nelson DM (1993) Separation and preconcentration of actinides from acidic media by extraction chromatography. Anal Chim Acta 281:361–372. https://doi.org/10.1016/0003-2670(93)85194-O
Marchesani G, Trotta G, De Felice P, Bortone N, Damiano R, Nicolini M, Accettulli R, Eugenio Chiaravalle AE, Iammarino M (2022) Fast and Sensitive Radiochemical Method for Sr-90 determination in food and feed by chromatographic extraction and liquid scintillation counting. Food Anal Methods 15:1521–1534
Swearingen KJ, Wall NA (2019) Fast and accurate simultaneous quantification of strontium-90 and yttrium-90 using liquid scintillation counting in conjunction with the Bateman equation. J Radioanal Nucl Chem 320:71–78
U.S. Environmental Protection Agency (2011) Rapid radiochemical method for total radiostrontium (Sr-90) in water for environmental remediation following homeland security events. EPA 402-R-10-001d. Accessed 31 Oct 2023
Wei C, Garnick K, Scott T, Wetherby A (2019) Simple methods for calculating activity of a parent-progeny system. J Radioanal Nucl Chem 322:263–269
Gibson JAB (1980) Modern techniques for measuring the quench correction in a liquid scintillation counter. In: Peng CT, Horrocks DL, Alpen EL (eds) Liquid scintillation counting, recent applications and developments. Academic Press, New York, pp 153–172
LNE-LNHB/CEA (2012) Nuclear data-210Pb. http://www.lnhb.fr/nuclides/Pb-210_tables.pdf. Accessed 31 Oct 2023
Acknowledgements
The CEA LASE team thanks the LA2D/LABOS project for the funding provided. The authors would like to thank Kimberly Colas for her valuable proofreading and corrections in English language.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baudat, E., Gautier, C., Bagán, H. et al. Optimization of a new radiochemical method based on extraction chromatographic resins and plastic scintillation for measurement of 90Sr in nuclear waste. J Radioanal Nucl Chem 333, 1911–1925 (2024). https://doi.org/10.1007/s10967-024-09396-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-024-09396-8