Abstract
In this study, the effect of the amount of maleic anhydride grafted polypropylene (PP-g-MA) (0–10 by weight %) compatibilizer on the thermal and mechanical properties of polypropylene/post-consumer poly (ethylene terephthalate) (PP/rPET) (70/30 by weight %) blends are investigated by means of melt blending method. Post-consumer PET bottle waste was recycled by means of bottle-to-bottle recycling method. The interactions between the polymers of the blends and PP-g-MA were investigated by means of Fourier transform infrared spectrometer (FTIR). The scanning electron microscope (SEM) results showed that the interfacial interactions of the polymers of studied blends improved with the increasing amount of PP-g-MA. The effect of the amount of PP-g-MA on the mechanical properties of the blends were investigated by means of tensile and flexural tests. It was obtained from mechanical tests that the tensile strength, tensile modulus, flexural strength and flexural modulus increased while elongation at break decreased with the increasing amount of PP-g-MA. The best mechanical performances were obtained by compatibilized PP/rPET blends with a tensile modulus value of 1795 MPa and flexural modulus value of 2515 MPa. The effect of the amount of PP-g-MA on the thermal properties of the blends were investigated by means of differential scanning calorimeter (DSC) and heat deflection temperature (HDT) tests. It was observed that the crystallization temperature (Tc) and HDT of the blends increased with the increasing amount of PP-g-MA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Poly (ethylene terephthalate) (PET) is a semi-crystalline thermoplastic that is widely used in many applications such as automotive, textile and food packaging because of its good physical, chemical and mechanical properties [1,2,3]. The increase in the accumulation of post-consumer PET waste all over the world leads to various environmental and economic concerns [4, 5]. PET wastes are recycled by means of chemical and mechanical recycling methods to prevent their environmental problems [6, 7]. Nowadays, there is a growing interest in corresponding studies for the mechanical recycling of post-consumer PET wastes [8, 9]. Especially, bottle-to-bottle recycling increases the remolding potential of post-consumer PET wastes in many industrial applications [10].
The recycling method play an important role in that the intrinsic viscosity of the PET is not decreasing too much in comparison with a conventional mechanical recycling process [11]. Also, manufacturing beverage bottles from recycled PET (rPET) rather than pure PET reduces carbon footprint and contributes a cost reduction in plastic packaging [12, 13]. The thermal, mechanical and chemical degradations are a serious problem during the recycling because limiting reuse of rPET decreases the molecular weight and intrinsic viscosity. In general, the properties of rPET can be improved by adding various fillers and also by modification with the use of different methods [14]. The polyolefin/rPET blends prepared by using simple and economical methods have started to attract attention in recent years because of overcoming the shortcomings of mechanical recycling [15, 16].
Polypropylene (PP) is the most widely preferred polyolefin in many applications such as automotive, packaging, construction and textile [17]. The production market of PP is rapidly growing especially in the automotive industry due to its various properties such as light-weighting, low cost and easy processability [18, 19].
PP, which is a commercial thermoplastic, can be used in place of engineering plastics when processed with various additives and fillers [20, 21]. PP blends prepared by physical compounding with at least one different polymer without chemical interaction play an important role in innovative and sustainable applications [22]. Accordingly, PP/rPET blends stand out due to their properties such as low cost, high performance and recyclability [23]. Recycling of post-consumer PET wastes with PP by a feasible method can be a gain for various plastic industries [24].
PP and rPET are immiscible during blending due to the differences in the chemical structure and polarity of the polymers. The immiscibility of PP/rPET blends leads to phase separation and poor interfacial properties between the components in the blends [25, 26]. Poor interfacial properties of immiscible blends have an adverse effect on the mechanical performance. The interfacial properties of PP/rPET blends are generally improved by means of compatibilizing with copolymers having different functional groups such as maleic anhydride (MA), acrylic acid (AA) and glycidyl methacrylate (GMA) [27, 28]. PP-g-MA is a very commonly used compatibilizing agent for improving miscibility in polyolefin blends [29]. Coupling process during compatibilization occurs by the interaction of the hydroxyl (-OH) and carboxyl (-COOH) functional end groups of PET in PP/rPET blends [30, 31]. Mi et al. [32] determined that the phase separation of blends was partially eliminated and the interfacial tension of the components of the blends was reduced when PP/PET blends were compatibilized with maleic anhydride functionalized copolymers [33]. The interfacial properties of PP/PET blends may be changed with subject to parameters such as process conditions, the amounts of compatibilizer and components of blends. Papadopoulou and Kalfoglou [34] used three types of SEBS-g-MA [35], PP-g-MA [36, 37] and LLDPE-g-MA copolymers [38] having maleic anhydride functional groups for the compatibilization of PP/PET blends. It was reported that PP-g-MA was more effective in comparison with the compatibilizers of SEBS-g-MA and LLDPE-g-MA in improving the interfacial properties of PP/PET blends. Yi et al.[39] reported that compatibilizing of PP/PET blends led to a reduced interfacial tension between PP and PET phases resulting in more homogeneous blends [40]. The type and the amount of compatibilizer have significant effects on the mechanical, morphological, rheological and thermal properties of PP/PET blends [41]. Lima et al. [42] determined that the immiscibility of PP/PET blend was eliminated with the increasing amount of compatibilizer and that changed the crystallization behavior of PP. Jahani et al. [43] reported that the size of the dispersed polymer phase in the matrix was reduced and more homogeneous PP/rPET blends were attained with the increasing amount of compatibilizer. Taepaiboon et al. [44] indicated as a result of their studies that PP/rPET blends compatibilized with 2–7 wt.% PP-g-MA had higher tensile strengths compared to pure PP. Friedrich et al. [45] reported that the improvement in the fiber-matrix adhesion properties of PP/PET blends compatibilized with ethylene glycidyl methacrylate improved the mechanical properties of the blends. Razak et al. [46] determined that the flexural and tensile strengths of PP/PET blends were improved when the blends were compatibilized with the maleic anhydride functionalized copolymer. Song and Pang[47]reported that the weight fraction of interphase and tensile strength increased by addition of the compatibilized PP/PET (75/20 wt. %) blends. Inoya et al. [48] reported that the presence of 5 wt.% compatibilizer was very effective in improving the mechanical properties of PP/rPET blends prepared using PET bottle wastes. Nonato and Bonse [49], investigated PP/rPET composites with the addition of PP-g-MA as a compatibilizer that the tensile strength of the composites increased with the increasing amount of compatibilizer due to the increase of adhesion between the PP matrix and recycled PET fiber.
There are various studies in literature investigated the effect of the process parameters, compatibilizer type and amount of compatibilizer on the morphological [50], thermal [51] and mechanical [52] properties of PP/PET blends. However, there seems to be limited number of studies on the preparation of PP/rPET blends in preceding literature [53]. The aim of this study is to improve the thermal and mechanical properties of PP blends prepared by using bottle-to-bottle recycled post-consumer PET (rPET) and by the inclusion of PP-g-MA compatibilizer. The blends were mixed with a weight percentage ratio of 70/30 (wt. %) throughout this study by melt blending method. In addition, isotactic polypropylene was used in the preparation of the blends. The blends were injection molded after being prepared in a twin-screw extruder in the presence of various amounts of PP-g-MA. The effects of amounts of PP-g-MA on the thermal stability behaviors, crystallization behaviors, microstructures, tensile and flexural behaviors of the blends were investigated. While examining the effect of compatibilizer on the properties of the prepared PP/rPET blends, the results were also compared with the PP/PET blends.
Experimental
Materials
Bottle grade PET with the commercial name of RAMAPET N1 having an intrinsic viscosity of 0.80 dl/g and melting point of 247 °C was supplied by Indorama Ventures PET Inc., Turkey. rPET was supplied by Kaptan Recycling Company, Turkey. PP with the commercial name of PETOPLAN MH-418 having a melt flow rate of 4.5 g/10 min (at 230 °C and 2.16 kg), melting point of 163 °C and density of 0.905 g/cm3 was supplied by PETKIM Inc., Turkey. PP-g-MA was supplied by Acar Chemicals Inc., Turkey. Antioxidant with the commercial name of Irganox 1010® having a density value of 1.15 g/cm3 in addition to the heat stabilizer with the commercial name of Irgafos 168® having a density value of 1.03 g/cm3 were supplied by Ciba Specialty Chemicals Inc., Switzerland.
Preparation of Blends
The post-consumer PET waste is recycled by means of bottle-to-bottle recycling method. The PET granules were prepared by twin screw extruder at temperatures of 250, 260, 270 °C by melt blending method. rPET and PET granules were first dried under vacuum at 100 °C for 4 h. Prior to the melt blending process, the components of the blends (PET, rPET, PP, PP-g-MA, antioxidant and heat stabilizer) were pre-mixed according to the composition given in Table 1. In addition, 0.2% (by weight) antioxidant and heat stabilizer were used in the preparation of all blends. A co-rotating twin screw extruder (D = 16 mm, L/D = 24; Gulnar, Turkey) was used for the preparation of the blends. The blend granules were prepared by twin screw extruder at screw speed of 200 rpm and temperatures of 260, 250, 240 °C by melt blending method. Before the molding process, the blend granules were dried under vacuum at 80 °C for 4 h. The PP/PET and PP/rPET granules were molded using Engel/Spex Victory 80 model injection moulding machine with an injection speed of 65 mm/s and molding clamp force of 300 kN at temperatures of 265, 250, 240 and 230 °C, respectively. Preparation procedure and set-up of PP/rPET blends are shown in Fig. 1.
Characterization of Blends
Fourier transform infrared spectrometer (FTIR) analysis of blends were carried out by Perkin Elmer Spectrum 100 brand ATR-FTIR device. FTIR analysis of PP/PET and PP/rPET blends were performed in order to observe the interactions between functional groups of PP-g-MA and the end groups of polymers.
The effect of the amount of PP-g-MA on the interfacial properties of the blends was investigated by Inspect/S50 model scanning electron microscope (SEM). The surfaces of tensile fractured samples of the blends were examined for SEM analysis. The samples were coated with gold under vacuum prior to the analysis to prevent arching.
The effect of the amount of PP-g-MA on the mechanical properties of the blends was investigated by tensile and flexural tests (Fig. 2). The tensile tests of the blends were conducted at a rate of 50 mm/min at room temperature by means of Zwick/Roell model universal testing device with 20 kN load cell according to ISO 527 standard. The flexural tests of the blends were also carried out by Zwick/Roell model three-point bending device at room temperature according to ISO 178 standard.
Heat deflection temperature (HDT) and differential scanning calorimeter (DSC) analysis of blends were performed for determining the thermal properties of the blends. HDT analysis of blends were carried out according to ISO 75 A standard using Instron/Ceast HV3 model device (1.8 MPa, 10 °C/min). SII Nanotechnology ExStar 7020 model DSC device was used for performing the melting and crystallization flow analyses of the blends. The samples were first heated from 25 °C to 280 °C under nitrogen atmosphere at a heating rate of 10 °C/min and kept at 280 °C for 5 min to erase the thermal history. Then, the samples were reheated from 25 °C to 280 °C at a heating rate of 10 °C/min after being cooled down from 280 °C to 25 °C at a cooling rate of 10 °C/min. Percent crystallization (Xc, %) of PP in the blends was calculated according to the relationship given in Eq. 1 using the data for the melting enthalpy of the polymer (ΔHm), weight fraction of the polymer in the blend (∅) and melting enthalpy of the polymer at 100% crystalline phase (ΔH°m). The thermal data were obtained from the second melting run of the DSC thermograms. The melting enthalpy of 100% crystalline PP is 209 J/g [54]. The melting enthalpy of 100% crystalline PET is 140 J/g.
Results and Discussion
Structural Properties of Blends
FTIR was used to monitor the interactions between the -OH and -COOH end groups of rPET and PP-g-MA (Fig. 3). The characteristic peaks of rPET and PET were also investigated according to peak of; -CH2 stretching at 2970 cm−1, C = O stretching at 1719 cm−1 [55], C = C bond stretching at 1580 cm−1, C–C stretching at 1410 cm−1 and -CH2 rocking at 720 cm−1. The characteristic peaks of pure PP were investigated according to peak of, bending of methyl groups at 1455–1370 cm−1, asymmetric aliphatic -CH2 stretching at 2918 cm−1, symmetric –CH stretching at 2838 cm−1, asymmetric -CH3 stretching at 2970 cm−1 and symmetric -CH2 stretching at 2868 cm−1. The weak peak intensity of the carbonyl (C = O) group of the ester group at 1719 cm−1 indicates that the PP/PET and PP/rPET blends are immiscible [56]. The increase in peak intensity at 1719 cm−1 is an indication that there are interactions between the maleic anhydride group of PP-g-MA and the functional end groups of rPET and PET [57]. It is implied that PP-g-MA copolymer is an effective compatibilizer for the current blends studied.
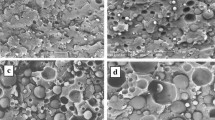
The SEM micrographs of tensile fractured surfaces of the pure PP, PP/PET and PP/rPET blends are shown in Fig. 4. Rupture related fibrillation is observed in the micrograph of pure PP (Fig. 4a). It was observed that the PP/PET and PP/rPET blends display different microstructural geometry. The dimension, shape and distribution properties of PET and rPET in the PP matrix were observed to be different in both compatibilized and uncompatibilized blends. It was observed in uncompatibilized PP/PET blends that the interfacial interaction is quite poor for PP and PET polymers (Fig. 4b). The addition of PP-g-MA in the PP/PET blends leads to a decrease in the diameter of PET domains and to improve the interfacial adhesion between PP and PET phases. It is related to the PET phase being distributed more homogeneously in PP matrix. It was observed in PP/PET blends that PET droplet size decreased with the increasing amount of PP-g-MA. The interfacial properties of PP/PET blends improved with compatibilization [58].
It was observed in uncompatibilized PP/rPET blends that rPET was distributed in the PP matrix in fibril form and that the interfacial properties are poor (Fig. 4g). This proves that the PP/PET and PP/rPET blends were immiscible blends. It was observed due to the poor interfacial interaction of PP/rPET blends that the rPET phase separates from the PP matrix thereby resulting in voids. The fact that the decrease of voids within the structure with the increasing amount of PP-g-MA is due to the improvement of the interfacial interaction between PP and rPET polymers. It is observed that the increase in the amount of PP-g-MA leads to a more homogeneous distribution of rPET in the PP matrix. Similar results have also been obtained in literature indicating that the presence of the compatibilizer changes the geometry of the PET phase and results in average particle size reduction. In the presence of the PP-g-MA, PP and rPET interaction was improved because of the hydrogen bonding interactions between the hydroxyl groups of the MA-g-PP and the end groups of the rPET (Fig. 4). It is concluded that the PP-g-MA enhances interfacial adhesion between two immiscible phases within the blends [59].
Mechanical Properties of Blends
The mechanical properties of PP/PET and PP/rPET blends have been determined by means of tensile and flexural tests [60]. The effect of the amount of PP-g-MA on the tensile behavior of the blends is shown in Fig. 5 and Fig. 6. The tensile test data are also given in Table 2. It has been observed that the tensile strength of uncompatibilized PP/PET and PP/rPET blends are lower than that of pure PP. This is due to the poor interfacial interaction between the polymers in PP/PET and PP/rPET blends. It was observed that the tensile strength of PP/PET and PP/rPET blends increased with the increasing amount of PP-g-MA [61]. It was observed from SEM micrographs evaluated together with the tensile test results that the results were compatible. This indicates that tensile properties are improved owing to the enhanced interfacial properties of the blends. The improvement of the interfacial properties between the two phases are resulted in a better stress transfer. The highest tensile strength and tensile modulus were obtained with PP/rPET/PP-g-MA10 and PP/PET/PP-g-MA5 blends (Fig. 5a, c). Generally, the increase in the amount of PP-g-MA in PP/PET blends were effectively enhanced the mechanical properties of the blends [62]. In our study, addition of PP-g-MA up to 5 wt. % tensile strength (36.1 MPa) and tensile modulus (1805 MPa) of the PP/PET blend increased. However, after the amount of PP-g-MA 5 wt. % they were a little decreased. These results were also supported by tensile stress–strain curves (Fig. 6) and SEM images (Fig. 4). While elongation at break was not significantly affected by PP-g-MA incorporation, tensile strength and modulus were noticeably improved. It is observed that pure PP exhibits higher elongation at break in comparison with that of PP/PET and PP/rPET blends. This is due to the brittle structure of PET additive in comparison with pure PP. The highest elongation at break of the PP/rPET blends are achieved with the addition of 10 wt.% PP-g-MA. The tensile strength of PP/PET blends improved when compatibilized with a copolymer.
The effect of the amount of PP-g-MA on the flexural behavior of PP/PET and PP/rPET blends are shown in Fig. 5. The flexural test data are also given in Table 2. It is observed that the flexural strength and flexural modulus of PP/PET and PP/rPET blends with the presence and absence of PP-g-MA are higher than that of pure PP. While the flexural strength of pure PP is 34.0 MPa, the flexural strength of uncompatibilized PP/PET and PP/rPET blends were obtained as 50.3 MPa and 48.3 MPa, respectively. This is due to the high flexural property of PET and rPET. It was observed that the flexural strength and flexural modulus of the blends were increased with the increasing amount of PP-g-MA (Fig. 5b, d). The flexural strength of the compatibilized blends were determined respectively to be 52.2 MPa and 50.3 MPa. The highest flexural strength was obtained for the blends compatibilized with the addition of 10 wt.% PP-g-MA. It was observed that the flexural strength and flexural modulus of PP/PET blends are greater in comparison with PP/rPET blends. The flexural strength and flexural modulus of PP/rPET blends improved with the increasing amount of compatibilizer [63]. This is due to the fact that the addition of PP-g-MA increases the interfacial interaction of rPET and PP polymers within the blends [64].
Thermal Properties of Blends
The effect of the amount of PP-g-MA on the melting and crystallization behavior of PP/PET and PP/rPET blends were investigated by DSC analysis. The second heating and cooling thermograms of pure PP and PP/PET and PP/rPET blends are shown in Fig. 7. The crystallization temperature (Tc), melting temperature (Tm), supercooling temperature (Tc-Tm), melting enthalpy (∆Hm) and crystallization percentage (Xc) of PP are shown in Table 3. It is observed from the cooling thermograms that the presence of PP-g-MA affects the crystallization temperature of PP. The Tc of PP in uncompatibilized PP/PET and PP/rPET blends are higher than pure PP. While the Tc of pure PP is 107.9 °C, the highest Tc of PP obtained is measured to be 122.0 °C in compatibilized PP/PET blends. This is due to the fact that PET and rPET phases distributed in PP act as a nucleating agent [65, 66]. Whereas the highest Tc of PP was obtained as 121.8 °C in compatibilized PP/rPET blends. A decrease is observed in the crystallization temperature of PP in PP/rPET blends with the increasing amount of PP-g-MA. The crystallization peak width of PP is observed to get narrower with the increasing amount of PP-g-MA. This is due to the fact that the polar maleic anhydride groups increase the interfacial interaction of rPET and PP polymers within PP/rPET blends. The increase in the crystallization temperature of PP reduces the injection molding cycle time as well as the product cost. Moreover, the fact that PET acts as a heterogeneous nucleating agent which further results in reduced supercooling temperature. It leads to a fast crystallization of PP during injection molding [67]. Nucleating agents increased the crystallization temperature of injection molded polymers while decreasing the supercooling temperature [68]. It is observed from the second heating thermograms that the presence of PP-g-MA affects the crystallization percentage of PP in the blends. The lowest crystallization percentage of PP in the PP/PET blend is obtained as 44.6% in the presence of 10 wt.% PP-g-MA. The lowest crystallization percentage in PP/rPET blend is obtained as 34.6% in the presence of 10 wt.% PP-g-MA. The crystallization percentage of PP in PP/rPET blends decreased more with the increasing amount of PP-g-MA in comparison with PP/PET blends. This is thought to be due to the restricted movement of PP chains and the different distribution variation of the rPET and PET phases in PP [69].
While melting temperature of PP in the blends was not significantly affected by PP-g-MA incorporation, crystallization temperature was noticeably increased. The melting behavior of PP was observed to be changing with the amount of PP-g-MA in second heating thermograms. It was observed that the Tm of PP in the blends was lower than pure PP. It was determined that the crystallization behavior of PP in the blends changed with the increasing amount of PP-g-MA [70].
The HDT of PP/PET and PP/rPET blends are shown in Table 3 and Fig. 8. While the HDT of pure PP is 57.4 °C, the HDT of PP/PET and PP/rPET blends are determined respectively as 64.4 °C and 64.6 °C. It is observed that the HDT increased by 8 °C in compatibilized blends in comparison with pure PP. This is considered to be due to the fact that both rPET and PET act as nucleating agent in the PP [49, 66]. The results demonstrate that the addition of rPET significantly improves the thermal performance of PP. The highest HDT in PP/PET and PP/rPET blends is obtained with the addition of 5 wt.% PP-g-MA. It is determined that the amount of PP-g-MA is not more effective than PET and rPET in improving the HDT of the blends. While it is observed that the HDT of the blends are close to each other, it is determined that rPET can be used instead of PET. Similar results were obtained with those in literature and it was observed that the thermal stability of PP improved by the preparation of compatibilized PP/rPET blends [71].
Conclusions
In this study, the effect of amount of PP-g-MA on the thermal and mechanical properties of PP/rPET blends (70/30) were investigated. FTIR analysis showed that the functional end groups of rPET exhibited an interaction with the maleic anhydride group of PP-g-MA. According to SEM micrographs, rPET phase dimensions decreased with the increasing amount of PP-g-MA and that the interfacial properties of the blends were improved. Mechanical test results showed that tensile modulus, tensile strength, flexural strength and flexural modulus increased due to the interfacial interaction between phases with the increasing amount of PP-g-MA. DSC results showed that the Tc of PP in the blends increased with the increasing amount of PP-g-MA compared to pure PP. The fact that the HDT of compatibilized PP/rPET blends being higher than pure PP showed that final PP products obtained had long-lasting shelf duration. In conclusion, it was observed that the addition of 5 wt% PP-g-MA was effective in improving the thermal and mechanical properties of PP/rPET blends and hence rPET can be used as an alternative sustainable material in place of bottle grade PET.
References
Awaja F, Pavel D (2005) Recycling of PET. Eur Polym J 41:1453–1477. https://doi.org/10.1016/j.eurpolymj.2005.02.005
Malik N, Kumar P, Shivastawa S, Ghosh SB (2017) An overview on PET waste recycling for application in packaging. Int J Plastics Technol 21:1–24. https://doi.org/10.1007/s12588-016-9164-1
Kangavar ME, Lokuge W, Manalo A, Karunasena W, Frigione M (2022) Investigation on the properties of concrete with recycled polyethylene terephthalate (PET) granules as fine aggregate replacement. Case Studies in Construction Materials 16:e00934. https://doi.org/10.1016/j.cscm.2022.e00934
Welle F (2011) Twenty years of PET bottle to bottle recycling-an overview. Resour Conserv Recycl 55:865–875. https://doi.org/10.1016/j.resconrec.2011.04.009
Zanela TMP, Muniz EC, Almedia CAP (2018) Chemical recycling of poly(ethylene terephthalate) (PET) by alkaline hydrolysis and catalyzed glycolysis Orbital-the electronic. J Chemistry 10:226–233. https://doi.org/10.17807/orbital.v10i3.1104
Asensio M, Nunez K, Guerrero J, Herrero M, Merino JC, Pastor JM (2020) Rhelogical modification of recycled poly(ethylene terephthalate): Blending and reactive extrusion. Polym Degrad Stab 179:109258. https://doi.org/10.1016/j.polymdegradstab.2020.109258
Dandekar Y, Kumar RS, Rajput MS (2020) Compressive property of newly developed composite material from polyethylene terephthalate (PET) waste and mild steel powder. Mater Today-Proc 27:83–86. https://doi.org/10.1016/j.matpr.2019.08.241
Raheem AB, Noor ZZ, Hassan A, Abd Hamid MK, Samsudin SA, Sabeen AH (2019) Current developments in chemical recycling of post-consumer polyethylene terephthalate wastes for new materials production: A review. J Clean Prod 225:1052–1064. https://doi.org/10.1016/j.jclepro.2019.04.019
Liu B, Lu XM, Ju ZY, Sun P, Xin JY, Yao XQ, Zhou Q, Zhang SJ (2018) Ultrafast homogeneous glycolysis of waste polyethylene terephthalate via a dissolution-degradation strategy. Ind Eng Chem Res 57:16239–16245. https://doi.org/10.1021/acs.iecr.8b03854
Pinter E, Welle F, Mayrhofer E, Pechhacker A, Motloch L, Lahme V, Grant A, Tacker M (2021) Circularity Study on PET Bottle-To-Bottle Recycling. Sustainability 13:7370. https://doi.org/10.3390/su13137370
Nabid MR, Bide Y, Jafari M (2019) Boron nitride nanosheets decorated with Fe3O4 nanoparticles as a magnetic bifunctional catalyst for post-consumer PET wastes recycling. Polym Degrad Stab 169:108962. https://doi.org/10.1016/j.polymdegradstab.2019.108962
Brock A, Williams IR (2020) Life cycle assessment of beverage packaging. Detritus 13:47–61. https://doi.org/10.31025/2611-4135/2020.14025
Kuczenski B, Geyer R (2010) Material flow analysis of polyethylene terephthalate in the US, 1996–2007. Resour Conserv Recycl 54:1161–1169. https://doi.org/10.1016/j.resconrec.2010.03.013
Al-Salem SM, Lettieri P, Baeyens J (2010) The valorization of plastic solid waste (PSW) by primary to quaternary routes: From re-use to energy and chemicals. Prog Energy Combust Sci 36:103–129. https://doi.org/10.1016/j.pecs.2009.09.001
Khan ZI, Habib U, Mohamad ZB, Raji AM (2021) Enhanced mechanical properties of a novel compatibilized recycled polyethylene terephthalate/polyamide 11 (rPET/PA11) blends. Express Polym Lett 15:1206–1215. https://doi.org/10.3144/expresspolymlett.2021.96
Latko-Duralek P, Dydek K, Boczkowska A (2019) Thermal, rheological and mechanical properties of PETG/rPETG blends. J Polym Environ 27:2600–2606. https://doi.org/10.1007/s10924-019-01544-6
Almajid A, Watter R, Kross T, Junaedi H, Gurka M, Khalil KA (2021) The multiple uses of polypropylene / polyethylene terephthalate microfibrillar composite structures to support waste management composite processing and properties. Polymers 13:1296. https://doi.org/10.3390/polym13081296
Ghanbari A, Seyedin S, Haddadi SA, Nofar M, Ameli A (2021) Reinforcing potential of recycled carbon fibers in compatibilized polypropylene composites. J Polym Res 28:145. https://doi.org/10.1007/s10965-021-02506-0
Adekunle AS, Adeleke AA, Sam Obu CV, Ikubanni PP, Ibitoye SE, Azeez TM (2020) Recycling of plastics with compatibilizer as raw materials for the production of automobile bumper. Cogent Eng 7:1801247. https://doi.org/10.1080/23311916.2020.1801247
Thenepalli T, Jun AY, Han C, Ramakrishna C, Ahn JW (2015) A strategy of precipitated calcium carbonate (CaCO3) fillers for enhancing the mechanical properties of polypropylene polymers. Korean J Chem Eng 32:1009–1022. https://doi.org/10.1007/s11814-015-0057-3
Kaushal A, Singh V (2020) Effect of filler loading on the shielding of electromagnetic interference of reduced graphene oxide reinforced polypropylene nanocomposites prepared via a twin-screw extruder. J Mater Sci Mater Electronics 31:22162–22170. https://doi.org/10.1007/s10854-020-04719-3
Huang HX, Huang HL (2021) Shortening droplet contact time over a wider impact velocity range by molding flexible nanohairs and substrates. ACS Appl Polym Mater 3:5749–5757. https://doi.org/10.1021/acsapm.1c01000
Kaymakci O, Uyanik N (2020) In-situ microfibrillar recycled PET/glass fiber PP/hybrid thermoplastic composites. Mater Plast 57:309–316. https://doi.org/10.37358/Mat.Plast.1964
Miandad R, Barakat MA, Aburiazaiza AS, Rehan M, Ismail IMI, Nizami AS (2017) Effect of plastic waste types on pyrolysis liquid oil. Int Biodeter Biodegr 119:239–252. https://doi.org/10.1016/j.ibiod.2016.09.017
Zdrazilova N, Haosnerova B, Kitano T, Saha P (2004) Rheological behavior of PP/PET and modified PP/PET blends. II. Dynamic viscoelastic properties. Polym Polym Compos 12:433–447. https://doi.org/10.1177/096739110401200508
Martin P, Devaux J, Legras R, van Gurp M, van Duin M (2001) Competitive reactions during compatibilization of blends of polybutylene phthalate with epoxide-containing rubber. Polymer 42:2463–2478. https://doi.org/10.1016/S0032-3861(00)00496-1
Pracella M, Pazzagli F, Galeski A (2002) Reactive compatibilization and properties of recycled poly(ethylene terephthalate)/polyethylene blends. Polym Bull 48:67–74. https://doi.org/10.1007/s00289-002-0001-7
Jung WC, Park KY, Kim JY, Suh KD (2003) Evaluation of isocyanate functional groups as a reactive group in the reactive compatibilizer. J Appl Polym Sci 88:2622–2629. https://doi.org/10.1002/app.11977
Chiu HT, Hsiao YK (2006) Compatibization of poly (ethylene terephthalate)/polypropylene blends with maleic anhydride grafted polyethylene-octene elastomer. J Polym Research 13:153–160. https://doi.org/10.1007/s10965-005-9020-z
Inuwa IM, Hassan A, Samsudin SA, Haafiz MKM, Jawaid M (2015) Interface modification of compatibilized polyethylene terephthalate/polypropylene blends: Effect of compatibilization on thermomechanical properties and thermal stability. J Vinyl Add Tech 23:45–54. https://doi.org/10.1002/vnl.21484
Zhu YL, Liang CS, Bo Y, Xu SA (2015) Compatibilization of polypropylene/recycled polyethylene terephthalate blends with maleic anhydride grafted polypropylene in the presence of diallyl phthalate. J Polym Research 22:35. https://doi.org/10.1007/s10965-014-0591-4
Mi D, Wang Y, Kuzmanovic M, Delva L, Jiang Y, Cardon L, Zhang J, Ragaert K (2019) Effects of phase morphology on mechanical properties: oriented/unoriented PP crystal combination with spherical/microfibrillar PET phase. Polymers 11:248. https://doi.org/10.3390/polym11020248
Tao YJ, Pan YX, Zhang ZS, Mai KC (2008) Non-isotherm crystallization, melting behavior and polymorphism of polypropylene in beta-nucleated polypropylene/recycled poly(ethylene terephthalate) blends. European Polym J 44:1165–1174. https://doi.org/10.1016/j.eurpolymj.2008.01.023
Papadopoulou CP, Kalfoglou NK (2000) Comparison of compatibilizer effectiveness for PET/PP blends: their mechanical, thermal and morphology characterization. Polymer 41:2543–2555. https://doi.org/10.1016/S0032-3861(99)00442-5
Araujo LMG, Morales AR (2018) Compatibilization of recycled polypropylene and recycled poly(ethylene terephthalate) blends with SEBS-g-MA. Polimeros 28:84–91. https://doi.org/10.1590/0104-1428.03016
Zander NE, Gillan M, Burckhard Z, Gardea F (2019) Recycled polypropylene blends as novel 3D printing materials. Addit Manuf 25:122–130. https://doi.org/10.1016/j.addma.2018.11.009
Khaledi B, Ebrahimi NG (2019) Structural analysis of poly (ethylene terephthalate) modified by polypropylene-graft-maleic anhydride from rheological data. J Appl Polym Sci 136:46896. https://doi.org/10.1002/app.46896
Kang TK, Kim Y, Lee WK, Park HD, Cho WJ, Ha CS (1999) Properties of uncompatibilized and compatibilized poly (butylene terephthalate) LLDPE blends. J Appl Polym Sci 72:989–997. https://doi.org/10.1002/(SICI)1097-4628(19990523)72:8%3c989::AID-APP2%3e3.0.CO;2-X
Yi X, Xu L, Wang Y-L, Zhong G-J, Ji X, Li Z-M (2010) Morphology and properties of isotactic polypropylene/poly (ethylene terephthalate) in situ microfibrillar reinforced blends: influence of viscosity ratio. Eur Polym J 46:719–730. https://doi.org/10.1016/j.eurpolymj.2009.12.027
Bae TY, Park KY, Kim DH, Suh KD (2001) Poly (ethylene terephthalate)/polypropylene reactive blends through isocyanate functional group. J Appl Polym Sci 81:1056–1062. https://doi.org/10.1002/app.1527
Park YW, Park M, Kim HY, Kim HC, Lim JC, Jin FL, Park SJ (2018) Thermal and curl properties of PET/PP blend fibres compatibilized with EAG ternary copolymer. Bull Mater Sci 41:104. https://doi.org/10.1007/s12034-018-1621-3
Lima MS, Matias ÁA, Costa JRC, Fonseca AC, Coelho JFJ, Serra AC (2019) Glycidyl methacrylate-based copolymers as new compatibilizers for polypropylene/polyethylene terephthalate blends. J Polym Res 26:127. https://doi.org/10.1007/s10965-019-1784-7
Jahani Y, Ghetmiri M, Vaseghi MR (2015) The effects of long chain branching of polypropylene and chain extension of poly (ethylene terephthalate) on thermal behavior, rheology and morphology of their blends. RSC Adv 5:21620–21628. https://doi.org/10.1039/C5RA00030K
Taepaiboon P, Junkasem J, Dangtungee R, Amornsakchai T, Supaphol P (2006) In situ microfibrillar-reinforced composites of isotactic polypropylene/recycled poly (ethylene terephthalate) system and effect of compatibilizer. J Appl Polym Sci 102:1173–1181. https://doi.org/10.1002/app.24402
Friedrich K, Evstatiev M, Fakirov S, Evstatiev O, Ishii M, Harrass M (2005) Microfibrillar reinforced composites from PET/PP blends: processing, morphology and mechanical properties. Compos Sci Technol 65:107–116. https://doi.org/10.1016/j.compscitech.2004.06.008
Razak NC, Inuwa IM, Hassan A, Samsudin SA (2013) Effects of compatibilizers on mechanical properties of PET/PP blend. Compos Interfaces 20:507–515. https://doi.org/10.1080/15685543.2013.811176
Song M, Pang YX (2001) Correlation among morphology interface, and mechanical properties: Experimental study. J Macromol Sci-Physics B40:1153–1167. https://doi.org/10.1081/MB-100107807
Inoya H, Leong YW, Klinklai W, Takai Y, Hamada H (2011) Compatibilization of recycled poly (ethylene terephthalate) and polypropylene blends: Effect of compatibilization on blend toughness, dispersion of minor phase, and thermal stability. J Appl Polym Sci 124:5260–5269. https://doi.org/10.1002/app.34385
Nonato RC, Bonse BC (2016) A study of PP/PET composites: Factorial design, mechanical and thermal properties. Polym Testing 56:167–173. https://doi.org/10.1016/j.polymertesting.2016.10.005
Cavus FK, Ozcanli Y, Beken M (2019) The effect of PET additive on mechanical and morphological properties of PP. J Fac Eng Archit Gazi Univ 34:319–326. https://doi.org/10.17341/gazimmfd.416494
Jayanarayanan K, Thomas S, Joseph K (2010) In situ microfibrillar blends and composites of polypropylene and poly (ethylene terephthalate): morphology and thermal properties. J Polym Res 18:1–11. https://doi.org/10.1007/s10965-009-9384-6
La Mantia FP, Titone V, Milazzo A, Ceraulo M, Botta L (2021) Morphology, rheological and mechanical properties of isotropic and anisotropic PP/rPET/GnP nanocomposite samples. Nanomaterials 11:3058. https://doi.org/10.3390/nano11113058
Techawinyutham L, Tengsuthiwat J, Srisuk R, Techawinyutham W, Rangappa SM, Siengchin S (2021) Recycled LDPE/PETG blends and HDPE/PETG blends: mechanical, thermal, and rheological properties. J Mater Res Technol JMR&T 15:2445–2458. https://doi.org/10.1016/j.jmrt.2021.09.052
Bahar E, Ucar N, Onen A, Wang Y, Öksüz M, Ayaz O, Ucar M, Demir A (2012) Thermal and mechanical properties of polypropylene nanocomposite materials reinforced with cellulose nano whiskers. J Appl Polym Sci 125:2882–2889. https://doi.org/10.1002/app.36445
Sriromreun P, Petchsuk A, Opaprakasit M, Opaprakasit P (2014) Miscibility and hydrolytic degradability of polylactic acid/poly (ethylene terephthalate-co-lactic acid) blends. Chiang Mai J Sci 41:691–703
Chen Z, Hay JN, Jenkins MJ (2012) FTIR spectroscopic analysis of poly (ethylene terephthalate) on crystallization. Eur Polym J 48:1586–1610. https://doi.org/10.1016/j.eurpolymj.2012.06.006
Mirjalili F, Moradian S, Ameri F (2013) Enhancing the dye ability of polypropylene fibers by melt blending with polyethylene terephthalate. Sci World J 2013:468542. https://doi.org/10.1155/2013/468542
Zhang XL, Li B, Wang K, Zhang Q, Fu Q (2009) The effect of interfacial adhesion on the impact strength of immiscible PP/PETG blends compatibilized with triblock copolymers. Polymer 50:4737–4744. https://doi.org/10.1016/J.POLYMER.2009.08.004
Lohar G, Tambe P, Jogi B (2022) Migration induced localization of multiwalled carbon nanotubes in PP phase of PP/ABS blend and their hybrid composites: Influence on mechanical and thermal properties. Mater Today Proc 49:1215–1224. https://doi.org/10.1016/j.matpr.2021.06.291
Tariq A, Ahmad NM, Abbas MA, Shakir MF, Khalid Z, Rafiq S, Ali Z, Elaissari A (2021) Reactive extrusion of maleic-anhydride grafted polypropylene by torque rheometer and its application as compatibilizer. Polymers 13:495. https://doi.org/10.3390/polym13040495
Koysuren O, Yesil Bayram G (2010) Effect of solid state srinding on properties of PP/PET blends and their composites with carbon nanotubes. J Appl Polym Sci 118:3041–3048. https://doi.org/10.1002/app.32727
Chiu H-T, Chuang C-Y (2008) Mechanical properties and compatibility of sulfonated poly(ethylene terephthalate)/cyclic olefin copolymer/maleic anhydride-grafted polyethylene-octene elastomers. Polym Plast Technol Eng 47:649–654. https://doi.org/10.1080/03602550802129478
Matias AA, Lima MS, Pereira J, Pereira P, Barros R, Coelho JFJ, Serra CA (2021) Use of recycled polypropylene/poly (ethylene terephthalate) blends to manufacture water pipes: An industrial scale study. Waste Manage 101:250–258. https://doi.org/10.1016/j.wasman.2019.10.001
Wang CG, Dai W, Zhang ZS, Mai KC (2013) Effect of blending way on beta-crystallization tendency of compatibilized beta-nucleated isotactic polypropylene/poly(ethylene terephthalate) blends. J Therm Anal Calorim 111:1585–1593. https://doi.org/10.1007/s10973-012-2425-0
Dehghani A, Ardekani SM, Al-Maadeed MA, Hassan A, Wahit MU (2013) Mechanical and thermal properties of date palm leaf fiber reinforced recycled poly (ethylene terephthalate) composites. Mater Des 52:841–848. https://doi.org/10.1016/j.matdes.2013.06.022
Ekinci A, Öksüz M, Ates M, Aydin I (2022) Polypropylene/postconsumer recycled poly (ethylene terephthalate) hybrid composites: evaluation of morphological, mechanical, thermal and electrical properties. In press, Iranian Polymer Journal
Wang D, Luo F, Shen Z, Wu X, Qi Y (2017) A study on the crystallization behavior and mechanical properties of poly (ethylene terephthalate) induced by chemical degradation nucleation. RSC Adv 7:37139–37147. https://doi.org/10.1039/C7RA06823A
Paszkiewicz S, Szymczyk A, Pawlikowska D, Irska I, Taraghi I, Pilawka R, Gu J, Li X, Tu Y, Piesowicza E (2017) Synthesis and characterization of poly (ethylene terephthalate-co-1,4-cyclohexanedimethylene terephtlatate)-block-poly (tetramethylene oxide) copolymers. RSC Adv 7:41745. https://doi.org/10.1039/C7RA07172H
Zhu Y, Liang C, Bo Y, Xu S (2015) Non-isothermal crystallization behavior of compatibilized polypropylene/recycled polyethylene terephthalate blends. J Therm Anal Calorim 119:2005–2013. https://doi.org/10.1007/s10973-014-4349-3
Jafari SH, Kalati-vahid A, Khonakdar HA, Asadinezhad A, Wagenkrecht U, Jehnichen D (2012) Crystallization and melting behavior of nanoclay-containing polypropylene/poly (trimethylene terephthalate) blends. Express Polym Lett 6:148–158. https://doi.org/10.3144/expresspolymlett.2012.16
Chaiwutthinan P, Suwannachot S, Larpkasemsuk A (2018) Recycled poly (ethylene terephthalate)/polypropylene/wollastonite composites using PP-g-MA as compatibilizer: Mechanical, thermal and morphological properties. J Metals Mater Minerals 28:115–123. https://doi.org/10.14456/jmmm.2018.34
Acknowledgements
This research was funded by Scientific Research Project Coordination Department of Yalova University, grant number “2018/DR/0005”. Aysun Ekinci thanks to the Scientific and Technological Research Council of Turkey (TUBITAK) for 2211/C scholarship program and the Council of Higher Education (YOK) for 100/2000 PhD scholarship program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
This paper is approved by all authors and there is no conflict of interest for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ekinci, A., Öksüz, M., Ates, M. et al. Thermal and Mechanical Properties of Polypropylene/Post-consumer Poly (ethylene terephthalate) Blends: Bottle-to-Bottle recycling. J Polym Res 29, 433 (2022). https://doi.org/10.1007/s10965-022-03229-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-022-03229-6