Abstract
Eugenol, a relatively cheap and abundant renewable resource, is used to design terminal diene compounds. Thiol-ene click reactions between the terminal diene intermediates and 1,6-hexanedithiol afford the corresponding oligomers with molecular mass of about 1.3 kDa. Finally, the oligomers and tricapto compound trimethylolpropane tris(3-mercaptopropionate) undergo thiol-oxidation reactions to form disulfide-crosslinked polymers. The brown polymers indicate self-healable behavior under UV light irradiation, presumably due to reversible reshuffling of weak disulfide bonds and/or reversible addition-dissociation reaction between benzoxazine and thiol groups.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Resource and energy crisis has been a global problem due to strong dependence of human life on fossil resources that become more and more deficient. Therefore, importance of renewable natural resources is emphasized in recent years by both scientists and engineers [1–8]. Eugenol (i.e., 4-allyl-2-methoxy phenol), a major component of clove essential oil, is a relatively cheap natural feedstock [9]. This compound has been successfully used in flavoring and pharmaceutical industry because of its pleasant odor. Besides, applications of eugenol in preparing polymer materials also draw great attention in recent years, because this renewably unique compound and its derivatives can be used as aromatic monomers [10–13]. Polymers from eugenol can usually endure high temperatures. For example, Muthusamy’s research group reported preparation of bio-based benzoxazine momomers by reactions between eugenol and different diamines [14]. After thermal curing of the benzoxazine monomers, the resulting polymers showed high thermal stability (340 °C) as well as good flame retardance. More recently, Deng and coworkers prepared polymeric microspheres with diameters ranging 500–800 μm from eugenol derived methacrylate monomer by using suspension polymerization in aqueous media [15]. The reusable microspheres possess remarkably large oil absorbency in a relatively high speed.
Self-healing or self-repairing phenomena are common in nature. For example, lost or injured tissues and organs can be regenerated or remended in most living organisms. Inspired by these phenomena, studies on artificial self-healing materials have attracted much interest in the past two decades [16–19]. Intrinsic and extrinsic self-healing materials are the two main types of the matter, and the former is more promising as these materials can be self-healed for many times under certain stimuli or even without external stimuli [20]. Among various intrinsic self-healing polymers, sulfur-containing materials such as sulfides, disulfides and trithiocarbonates are intensively researched due to relatively easy accessibility to weak sulfur-sulfur and carbon-sulfur bonds, and these bonds can be reversibly formed and cleaved under light, heating or redox stimuli [21–24]. Well known routes to carbon-sulfur and sulfur-sulfur bonds are thiol-ene and thiol-oxidation reactions [25, 26]. A typical example is the work of Canadell and his colleagues reported in 2011 [27]. They utilized a disulfide-bond-containing epoxy compound and a four-thiol-group species to undergo ring-opening reactions between thiol and epoxy groups. The resulting crosslinked material, after being cut, showed self-healing behavior at 60 °C for 1 h. This self-healing property may origin from reversible exchange of disulfide groups in the polymer. Recently, our research group designed a photo-active trithiocarbonate that can be a crosslinker of multi-hydroxyl polymer, and the crosslinked polymer indicated self-healing property under UV irradiation because of weak C-S bonds in the polymer can be reversibly reshuffled under light stimulus [28].
Herein, we report preparation of self-healing polymers from the renewable eugenol by combining thiol-ene and thiol-oxidation reactions. Thiol-ene/yne click reactions have been extensively studied in recent years [29, 30]. To the best of our knowledge, there are no reports on preparation of self-healing functional polymers based on the cheap and renewable eugenol resource.
Experimental
Materials
1,4-Phenylenediamine, allylic bromide, 1,6-hexanedithiol, benzophenone, potassium carbonate, sodium carbonate, sodium thiosulfate, sodium iodide, trimethylolpropane tris(3-mercaptopropionate), acetone, dichloromethane (DCM), tetrahydrofuran (THF), 37 % aqueous HCHO, chloroform, iso-propanol and 30 % H2O2 were all AR grade and were used straightly as received from Shanghai Aladdin Co. Ltd. Eugenol (industrial grade) was purified on silica gel column prior to use.
Characterization
Gel permeation chromatography (GPC) was conducted on an HP 1100 HPLC, equipped with a Waters 2414 refractive index detector and three Styragel HR 2, HR 4, HR 5 of 300 × 7.5 mm columns (packed with 5 mm particles of different pore sizes). The column packing allowed the separation of polymers over a wide molecular weight range of 500–1,000,000. THF was used as the eluent at a flow rate of 1 mL.min−1 at 40 °C. PMMA standards were used as the reference. IR measurement was completed with FTIR-650 spectrometer by using KBr pellet pressing method. Structures of trithiocarbonate and the copolymer poly(MMA-co-HEA) were characterized by 1H NMR spectroscopy on a Bruker AV 400 MHz spectrometer. CDCl3 was used as the solvent. Thiol-ene reactions and self-healing experiments were conducted under 365 nm of UV irradiation by using a UV lamp (power 2500 mW.cm−2). The glass transition temperature (Tg) was tested by a differential scanning calorimeter DSC 2910(Modulated DSC, TA Instruments Corporation, USA). Tensile strength analysis was conducted with an electronic universal testing machine according National standard GB/T1040.2-2006. Impact strength was performed on an impact testing machine with the type of XCJ-40. SEM images were given from a Hitachi scanning electron microscope.
Synthesis of O-allylic eugenol (2)
To a 250 mL of flask were added sequentially eugenol (1.642 g, 10 mmol), allylic bromide (1.331 g, 11 mmol), potassium carbonate (1.382 g, 10 mmol), and acetone (20 mL). The mixture was stirred at room temperature for 24 h. After the reaction was completed, water was added to the mixture, followed by adding dichloromethane to extract product. The organic phase was dried with anhydrous sodium sulfate, and crude product was obtained after removal of solvent. Further purification of the crude product on silica gel chromatography with petroleum ether/ethyl acetate (5 : 1, v : v) as eluant gave oily 2 (1.14 g, 56 %). 1H NMR (400 MHz, CDCl3): δ 6.83–6.68 (m, 3H), 6.16–5.83 (m, 2H), 5.49–5.24 (m, 2H), 5.12–5.03 (m, 2H), 4.63–4.55 (m, 2H), 3.86 (s, 3H), 3.44–3.36 (m, 2H).
Synthesis of eugenol-based oligomer (3) via thiol-ene reaction
The allylic eugenol 2 (1.02 g, 5 mmol), 1,6-hexanedithiol (1.127 g, 7.5 mmol), and benzophenone (0.01 g) were dissolved in dichloromethane (10 mL). The solution was irradiated under UV light for 3 h. Solvent and excess monomers were removed under reduced pressure, and the residue was crude oligomer 3 as a viscous liquid (0.785 g, 77 % conversion based on eugenol).
Preparation of eugenol-derived crosslinked polymer (5)
The oligomer 3 (4.0 g), crosslinker trimethylolpropane tris(3-mercaptopropionate) (4) (20 mg), NaI (10 mg) and 30 % H2O2 (2 mL) were dissolved in THF (40 mL). The resulting solution was stirred at room temperature till gel appeared, then aqueous 5 % Na2S2O3 (10 mL) was poured into the mixture, and stirring was continued. When yellow color disappeared, the system was stirred for another 1 h. After filtration and drying, the polymer 5 was obtained as a light brown gel-like semi-solid, which can form a sticky film on glass substrates.
Synthesis of eugenol-derived benzoxazine (6)
To a 250 mL of flask were added sequentially eugenol (3.28 g, 20 mmol), 1,4-phenylenediamine (1.08 g, 10 mmol) and 37 % aqueous formaldehyde (3.25 g, 40 mmol). The mixture was stirred at 120 °C for 40 min. When the reaction was completed, chloroform (20 mL) was added, and the mixture was washed twice with saturated aqueous Na2CO3. The organic phase was dried with anhydrous Na2SO4, filtered, and the filtrate was concentrated to afford crude product (4.0 g), which was recrystallized in iso-propanol gave benzoxazine product 6 (3.05 g, 63 %). M.p. 89–91 °C. 1H NMR (400 MHz, CDCl3): δ 7.14 (s, 4H), 6.60 (s, 2H), 6.46 (s, 2H), 5.95–5.85 (m, 2H), 5.42 (s, 4H), 5.20–5.15 (m, 2H), 4.56 (s, 4H), 3.98 (s, 6H), 3.31–3.26 (m, 4H).
Synthesis of eugenol-derived benzoxazine polymer via thiol-ene
The eugenol-derived benzoxazine monomer 6 (0.242 g, 0.5 mmol), 1,6-hexanedithiol (0.078 g, 0.52 mmol) and benzophenone (0.001 g) were dissolved in dichloromethane (10 mL). The solution was irradiated under UV for 3 h. Excess cold methanol was then poured into the reaction mixture to precipitate polymer product (0.21 g, 62 %). Molecular weight of the oligomer was Mn,GPC = 1307 from GPC.
Preparation of eugenol-derived benzoxazine crosslinked polymer (8)
In a similar method as preparation of the polymer 5, eugenol-derived benzoxazine crosslinked polymer 8 was obtained. Note that the precipitated 8 is at swell state and can form a film on glass substrates. But, compared with polymer 5, the film of 8 becomes relatively “hard” after being dried.
Measurement of chemical resistance of the eugenol-derived crosslinked polymer
The crosslinked polymer was formed a film on a glass substrate (mass of the film was weighed m1), which was then dipped into the corresponding media under ambient conditions. One week later, the sample was taken out of the media, dried, weighed (mass of the film was weighed m2) and observed the appearance as well as changes. Relative loss of mass of the film was calculated as △m% = [(m1 – m2)/m1] × 100 %. The smaller △m% is, the better chemical resistance of the polymer is.
Self-healing behavior of the eugenol-derived crosslinked polymer
The film of the crosslinked polymer was scratched by a razor. Then, the “injured” sample was exposed to UV (λ = 365 nm) light. After a period of time, changes of the sample are observed.
Results and discussion
Synthesis of O-allylic eugenol (2)
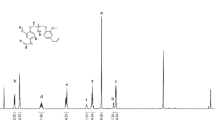
There exists an allylic group in eugenol molecule. It will become a diene if another allylic group is introduced. Thus, by using Williamson method for ether synthesis, eugenol reacts with allylic bromide under basic conditions to afford O-allylic eugenol (2) in moderate yield (Scheme 1). Structure of compound 2 is confirmed by 1H NMR spectrum shown in Fig. 1. Note that peaks at 0–2 ppm in Fig. 1 may be residues of water (c.a. 1.6 ppm) and petroleum ether, which have no negative effect on subsequent reactions such as thiol-ene.
Synthesis of eugenol-derived oligomer (3) via thiol-ene reaction
Thiol-ene reaction can proceed through radical or ionic route. For example, thia-Michael addition is usually through sulfur anion’s attack on an electron-deficient double bond [31]. On the other hand, in the presence of a radical initiator, thiol-ene reaction will proceed via radical species, a frequently utilized route to varieties of polymers and oligomers [32]. For our system, the eugenol-derived diene compound 2 can undergo thiol-ene reaction with 1,6-hexanedithiol in the presence of a benzophenone photo-initiator under UV irradiation conditions. After reaction for 3 h, the corresponding oligomer was obtained. 1H NMR spectrum indicates that vinyl signal (chemical shift of 5–6 ppm) almost disappear completely, meaning that the “ene” part of compound 2 is converted to saturated one (Fig. 2). To keep terminal groups to be mercapto ones, excess 1,6-hexanedithiol is added in the reaction system.
According to Fig. 2, the weak peak at chemical shift of 2.26 is signal of mecapto protons (Ha), and the number of these terminal protons is designated as two, which is base of other peaks. Thus, the number of aryl protons (Hb) is about 18, indicating that there are around six phenyl units (18 ÷ 3 = 6) in the oligomer. The number of Hc (c.a. 12) and Hd (c.a. 18) further prove that about six units exist in the oligomer. Therefore, molecular weight of the oligomer 3 can be estimated as Mn,NMR = 340 × 6 + 150 = 2190 Da. The molecular weight of 3 measured by GPC is Mn,GPC = 2280 Da, which is consistent with Mn,NMR. Molecular weight distribution of 3 is 1.66.
Preparation and characterization of eugenol-derived crosslinked polymer (5)
With oligomer 3 in hand, we then investigate crosslinking of 3 with trimethylolpropane tris(3-mercaptopropionate) (4) under oxidation conditions. It is known that thiol compounds can be oxidized by iodine or hydrogen peroxide to form the corresponding disulfide products [33]. Herein, reactions between 3 and 4 are conducted under hydrogen peroxide-NaI oxidation conditions, in which in situ formed iodine acts as an oxidant (Scheme 1). After reaction, the oligomer 3 is transformed into crosslinked polymer 5. IR spectrum of 5 is shown in Fig. 3. Absence of peaks at 2500–2600 cm−1 indicates that mercapto groups (−SH) in oligomer 3 has been consumed and transformed in to double sulfide groups (−S-S-). Furthermore, weak peaks at 400–500 cm−1 may prove the presence of double sulfide groups (−S-S-).
From IR spectrum of polymer 5, crosslinking is confirmed. What’s more, medium resistance of 5 is measured to estimate qualitatively crosslinking density. The results are shown in Table 1. The polymer shows good resistance to strong acid, a certain resistance to ethanol and toluene, and poor resistance to strong base and good solvent THF. These results indicate that crosslinking density is not high for the polymer 5.
Mechanical properties are important for materials. Thus, tensile strength of the polymer 5 is measured as 0.93 Mpa, elongation rate of rupture is 89 %, impact strength is 26 kJ.m−2. Hardness of the polymer film is lower than 2B from pencil method. Besides, glass transition temperature of the polymer 5 is −44.2 °C.
Next, we test self-healing behavior of the polymer under UV irradiation. The light brown film of polymer 5 is scratched by a razor to form the “injured” film as indicated in Fig. 4a. Then, the “injured” film is under 365 nm of UV irradiation. Ten minutes later, the “injured” film restore to its original state (Fig. 4b). The self-healing property may result from two aspects: one is that the “injured” part of the polymer becomes soft under thermal condition (heat from UV) to make the two sides (surfaces) of the scratch close to and reach each other; the other is that weak S-S bonds reversibly cleave and reform under UV irradiation conditions. To further observe self-healing behavior of the polymer, SEM image is given in Fig. 5. Similar changes are observed in Fig. 5, i.e., the scratched film was restored after 10 min of UV irradiation.
Preparation and characterization of eugenol-derived crosslinked polymer (8)
Benzoxazine polymers as novel phenolic resin have attracted considerable attention in recent years due to their advantages such as excellent heat resistance, flame retardance, high modulus and strength [34–36]. To extend application of eugenol and to improve performance of eugenol-derived polymers, the corresponding benzoxazine from eugenol is designed and synthesized as shown in Scheme 2. Firstly, eugenol, formaldehyde, and 1,4-phenylene diamine react to form benzoxazine compound 6. The structure of compound 6 is well characterized by 1H NMR spectrum as demonstrated in Fig. 6. Note that the peak at 1.57 ppm in Fig. 6 is the residue of water, and that at 1.26 ppm may be the residue of petroleum ether. These impurities will not affect subsequent reactions. Then, the benzoxazine compound 6 reacts with 1,6-hexanedithiol under photo-initiated radical polymerization conditions to produce the oligomer 7. Note that in oligomer 7, there still exists a certain amount of carbon-carbon double bonds according to 1H NMR spectrum (Fig. 7), which may origin from ring opening of benzoxazine ring by 1,6-hexanedithiol [37]. The weak peak at 2.26 ppm proves that there are mercapto groups in the oligomer 7. Molecular weight of 7 is measured as 1370 Da with polydispersity of 1.22. Finally, the oligomer 7 and trimethylolpropane tris(3-mercaptopropionate) (4) are oxidized to disulfide-bond-linked deep-brown polymer 8.
To prove the formation of 8 from 7, IR analysis are conducted as demonstrated in Fig. 8. There is a weak peak at 2570 cm−1 for IR spectrum of the oligomer 7, meaning the presence of mercapto groups (−SH). After oxidation reaction, the disappearance of the weak peak at 2570 cm−1 indicates mercapto groups are transformed into double sulfide groups.
Crosslinking density of the polymer 8 is estimated by measuring medium resistance as shown in Table 2. Similar to the results of polymer 5, the polymer 8 has good resistance to strong acid, a certain resistance to ethanol and toluene, and poor resistance to aqueous solution of strong base and good solvent THF. Thus, crosslinking density of 8 is not high.
Mechanical properties of the polymer 8 are also determined. Tensile strength of 8 is 0.76 Mpa, elongation rate of rupture is 78 %, and impact strength is 23 kJ.m−2. In comparison, mechanical properties of 8 are weaker than that of 5, which may be ascribed to the smaller molecular weight of the oligomer 7 than that of 3. For the thermal property of 8, its glass temperature is −48 °C.
Self-healing properties of 8 is studied in a similar manner as that of polymer 5. The film of polymer 8 is scratched with a razor to form “injured” polymer sample as shown in Fig. 9a. Then the “injured” sample is placed under UV irradiation. Ten minutes later, the scratched part of the polymer disappears, indicating that the polymer possesses self-healable properties. This behavior is also ascribed to reshuffling of weak S-S bonds in the polymer under light irradiation.
To further observe microscopic self-healing process of the eugenol-derived benzoxazine polymer 8, images from scanning electron microscope (SEM) are shown in Fig. 10. Figure 10a is an original film of polymer 8, which is then cut by a razor to for a “十” shape as indicated in Fig. 10b. Width of the crack is about 50–80 μm. The injured film is irradiated under UV light for 5 min, and the crack is almost self-healed (Fig. 10c). After UV irradiation for another 5 min, the film almost restore to its original state (Fig. 10d). One of advantages of reversible chemical bonds-induced self-healing is that self-healing behavior can be cycled for many times. Figure 11 shows three self-healing cycles of polymer 8. The polymer can be well healed under UV irradiation even after three cycles.
SEM images showing recyclable self-healing process of eugenol-derived benzoxazine polymer 8. (a) the cut sample with a “十” shape; (b) the cut sample after being irradiated for 10 min; (c) the sample was cut again at the same site; (d) the cut sample after being irradiated for 10 min; (e) the sample was cut for the 3rd time at the same site; (f) the cut sample after being irradiated for 10 min
Conclusions
Terminal diene intermediates are synthesized from renewable resource enugenol by using etherification and benzoxazine formation reactions, respectively. Then the intermediates undergo thiol-ene click reaction with 1,6-hexanedithiol under UV irradiation conditions. Molecular weights of the resulting oligomers are about 1300 Da, and hardness of the film is lower than 2B. The oligomers further react with tri-mercapto compound trimethylolpropane tris(3-mercaptopropionate) under oxidation conditions to form disulfide-bonds-crosslinked polymers. The brown crosslinked polymers show self-healing properties under UV light irradiation for 10 min, which may origin from reversible reshuffling of disulfide-bonds. This work is expected to extend applications of eugenol in designing functional materials.
References
Iwata T (2015) Biodegradable and bio-based polymers: future prospects of eco-friendly plastics. Angew Chem Int Ed 54:3210–3215
Yao KJ, Tang CB (2013) Controlled polymerization of next-generation renewable monomers and beyond. Macromolecules 46:1689–1712
Wilbon PA, Chu FX, Tang CB (2013) Progress in renewable polymers from natural terpenes, terpenoids, and rosin. Macromol Rapid Commun 34:8–37
Liu R, Zhang XP, Zhu JJ, Liu XY, Wang Z, Yan JL (2015) UV-curable coatings from multiarmed cardanol-based acrylate oligomers. ACS Sustain Chem Eng 3:1313–1320
Cheng CJ, Zhang X, Huang QH, Dou XQ, Li J, Cao XX, Tu YM (2015) Preparation of fully bio-based UV-cured non-isocyanate polyurethanes from ricinoleic acid. J Macromol Sci Part A Pure Appl Chem 52:485–491
Cheng CJ, Bai XX, Liu SJ, Huang QH, Tu YM, Wu HM, Wang XJ (2013) UV cured polymer based on a renewable cardanol derived RAFT agent. J Polym Res 20:197
Voirin C, Caillol S, Sadavarte NV, Tawade BV, Boutevinab B, Wadgaonkar PP (2014) Functionalization of cardanol: towards biobased polymers and additives. Polym Chem 5:3142–3162
Jahangiri M, Bagheri M, Farshi F, Abbasi F (2015) Optimized synthesis of hydroxypropyl cellulose-g-poly(ε-caprolactone) network. J Polym Res 22:196
Shibata M, Tetramoto N, Imada A, Neda M, Sugimoto S (2013) Bio-based thermosetting bismaleimide resins using eugenol, bieugenol and eugenol novolac. React Funct Polym 73:1086–1095
Dumasa L, Bonnauda L, Olivierb M, Poortemanb M, Dubois P (2015) Bio-based high performance thermosets: Stabilization and reinforcement of eugenol-based benzoxazine networks with BMI and CNT. Eur Polym J 67:494–502
Periyasamy T, Asrafali SP, Muthusamy S (2015) New benzoxazines containing polyhedral oligomeric silsesquioxane from eugenol, guaiacol and vanillin. New J Chem 39:1691–1702
Rojo L, Vazquez B, Parra J, Bravo AL, Deb S, Roman JS (2006) From natural products to polymeric derivatives of “eugenol”: a new approach for preparation of dental composites and orthopedic bone cements. Biomacromolecules 7:2751–2761
Qin JL, Liu HZ, Zhang P, Wolcott M, Zhang JW (2014) Use of eugenol and rosin as feedstocks for biobased epoxy resins and study of curing and performance properties. Polym Int 63:760–765
Thirukumaran P, Shakila A, Muthusamy S (2014) Synthesis and characterization of novel bio-based benzoxazines from eugenol. RSC Adv 4:7959–7966
Deng JP, Yang BW, Chen C, Liang JY (2015) Renewable eugenol-based polymeric oil-absorbent microspheres: preparation and oil absorption ability. ACS Sustain Chem Eng 3:599–605
Wei Z, Yang JH, Zhou JX, Xu F, Zrínyi M, Dussault PH, Osadag Y, Chen YM (2014) Self-healing gels based on constitutional dynamic chemistry and their potential applications. Chem Soc Rev 43:8114–8131
Benight SJ, Wang C, Tok JBH, Bao ZN (2013) Stretchable and self-healing polymers and devicesfor electronic skin. Prog Polym Sci 38:1961–1977
Billiet S, Hillewaere XKD, Teixeira RFA, Du Prez FE (2013) Chemistry of crosslinking processes for self-healing polymers. Macromol Rapid Commun 34:290–309
Nguyen LTT, Nguyen HT, Truong TT (2015) Thermally mendable material based on a furyl-telechelic semicrystalline polymer and a maleimide crosslinker. J Polym Res 22:186
Guimard NK, Oehlenschlaeger KK, Zhou JW, Hilf S, Schmidt FG, Barner-Kowollik C (2012) Current trends in the field of self-healing materials. Macromol Chem Phys 213:131–143
Scott TF, Schneider AD, Cook WD, Bowman CN (2005) Photoinduced plasticity in cross-linked polymers. Science 308:1615–1617
Michal BT, Jaye CA, Spencer EJ, Rowan SJ (2013) Inherently photohealable and thermal shape-memory polydisulfide networks. ACS Macro Lett 2:694–699
Amamoto Y, Kamada J, Otsuka H, Takahara A, Matyjaszewski K (2011) Repeatable photoinduced self-healing of covalently cross-linked polymers through reshuffling of trithiocarbonate units. Angew Chem Int Ed 50:1660–1663
Cash JJ, Kubo T, Bapat AP, Sumerlin BS (2015) Room-temperature self-healing polymers based on dynamic-covalent boronic esters. Macromolecules 48:2098–2106
Yu L, Wang LH, Hu ZT, You YZ, Wu DC, Hong CY (2015) Sequential Michael addition thiol–ene and radical-mediated thiol–ene reactions in one-pot produced sequence-ordered polymers. Polym Chem 6:1527–1532
Zaquen N, Wenn B, Ranieri K, Vandenbergh J, Junkers T (2014) Facile design of degradable poly(β-thioester)s with tunable structure and functionality. J Polym Sci Part A Polym Chem 52:178–187
Canadell J, Goossens H, Klumperman B (2011) Self-healing materials based on disulfide links. Macromolecules 44:2536–2541
Cheng CJ, Bai XX, Zhang X, Li HX, Huang QH, Tu YM (2015) Self-healing polymers based on a photo-active reversible addition-fragmentation chain transfer (RAFT) agent. J Polym Res 22:46
Truong VX, Dove AP (2013) Organocatalytic, regioselective nucleophilic “click” addition of thiols to propiolic acid esters for polymer–polymer coupling. Angew Chem Int Ed 52:4132–4136
Hoyle CE, Lowe AB, Bowman CN (2010) Thiol-click chemistry: a multifaceted toolbox for small molecule and polymer synthesis. Chem Soc Rev 39:1355–1387
Lin YM, Lu GP, Cai C, Yi WB (2015) An odorless thia-Michael addition using Bunte salts as thiol surrogates. RSC Adv 5:27107–27111
Xu JT, Boyer C (2015) Visible light photocatalytic thiol–ene reaction: an elegant approach for fast polymer postfunctionalization and step-growth polymerization. Macromolecules 48:520–529
Witt D (2008) Recent developments in disulfide bond formation. Synthesis 2491–2509
Men WW, Lu ZJ (2007) High performance polybenzoxazines. Prog Chem 19:779–786
Hao GP, Li WC, Qian D, Wang GH, Zhang WP, Zhang T, Wang AQ, Schüth F, Bongard HJ, Lu AH (2011) Structurally designed synthesis of mechanically stable poly(benzoxazine-co-resol)-based porous carbon monoliths and their application as high-performance CO2 capture sorbents. J Am Chem Soc 133:11378–11388
Calò E, Maffezzoli A, Mele G, Martina F, Mazzetto SE, Tarzia A, Stifani C (2007) Synthesis of a novel cardanol-based benzoxazine monomer and environmentally sustainable production of polymers and bio-composites. Green Chem 9:754–759
Kawaguchi AW, Sudo A, Endo T (2013) Polymerization–depolymerization system based on reversible addition-dissociation reaction of 1,3-benzoxazine with thiol. ACS Macro Lett 2:1–4
Acknowledgments
The work was financially supported by Natural Science Foundations of China (NO. 21264008, 21564004) and the 8th Foundation of Creativity and Study for College Students in Jiangxi Science Technology Normal University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheng, C., Zhang, X., Chen, X. et al. Self-healing polymers based on eugenol via combination of thiol-ene and thiol oxidation reactions. J Polym Res 23, 110 (2016). https://doi.org/10.1007/s10965-016-1001-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-016-1001-x