Abstract
Based on Diels-Alder reaction, a furyl-telechelic semicrystalline polycaprolactone was crosslinked by a tris-maleimide crosslinker. The synthesized precursors and network were fully characterized via proton nuclear magnetic resonance (1H NMR) and Fourier transform infrared (FT-IR) spectroscopies, gel permeation chromatography (GPC), thermogravimetric analysis (TGA), differential scanning calorimetry (DSC) and wide-angle powder X-ray diffraction (XRD) measurements. The obtained material showed mendability of scratches under thermal treatment, as evidenced by optical microscopy and tensile analysis. The mending process was a combination of the shape recovery effect favoring scratch closure and the re-crosslinking of the cleaved Diels-Alder bonds at temperatures slightly above the melting transition of polycaprolactone chains. A scratch healing efficiency determined by tensile tests of about 70 % was achieved.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Crosslinked polymers find broad applications as matrices for composites, foamed structures, structural adhesives, insulators for electronic packaging, anti-corrosion coatings, etc. Nevertheless, these materials are easily damaged when continuously exposed to the external environment, such as mechanical attack, UV radiation, or a combination of these factors. Small defects are easily created in the materials, but difficult or impossible to be detected and repaired. The presence of microcracks can adversly change the material final properties, and can even develop further into damage which considerably shortens material lifetime. Inspired by the self-healing phenomenon of biological organization, self-healing polymers have become an exciting research field in the study of thermosetting polymers in the past decades [1]. For thermosets, self-healing is challenging as the materials do not flow under the post-processing application of heat, as do thermoplastics.
In recent decades, various self-healing approaches have been developed rapidly, including encapsulation, hollow fibres, microvascular networks, supramolecular self-assembly, and reversible chemistry [2–4]. Among these approaches, intrinsic self-healing polymeric systems, which contain reversible covalent bonds, is a particularly attractive one. These polymer networks have high self-healing efficiency and repeatedly healing ability to heal the damage at the same position [5–7]. The most prominent mechanism is based on the Diels-Alder (DA) reaction between the furan and maleimide groups, which is thermally reversible [8–10]. When a DA adduct is heated to an appropriate temperature (above 90 °C), the retro-DA reaction takes place with rupture of the initially formed covalent bonds [11]. When cooled down to lower temperatures (below 90 °C), the DA reaction proceeds again and the covalent bonds reform [12].
Chen et al. [11] made use of multifuran/maleimide moieties that formed a reversible network. Because covalent bonds are much stronger than the bond between the DA adducts, cracks are more likely to propagate through the DA bonds than the other bonds. As a consequence, when a fissure appears, the recovery of broken DA crosslinks allows for the crack surfaces to be healed. Later on, other research groups have studied various polymeric systems bearing furan/maleimide functionalities as pendant groups. For instance, Tian et al. [13] attached furan/maleimide groups to epoxy resins. Zhang et al. [14] reported a polyketone containing a furan functionality that was crosslinked with a bismaleimide giving a recyclable thermoset. Kavitha et al. [15] synthesized a polymethacrylate bearing the furan functionality and crosslinked it using a bismaleimide crosslinker. Scheltjens et al. [16] studied flexible DA thermosets with different lengths of the furan and maleimide units. One of the recent strategies made use of two pendant furan and maleimide moieties on the same polymethacrylate backbone [17].
On the other hand, shape memory polymers have the capability of recovering their original shape by heating, even after large deformation [18–23]. Thus, the shape memory effect has been lately explored to replace the use of external force to bring the two crack surfaces together, so that further healing reactions can occur [24–30]. The approach combining the shape recovery feature and DA chemistry in a single polymeric material has recently been exploited in poly(tetramethylene oxide)-poly(p-dioxanone) co-network [31] and polyurethanes [32–34].
In this paper, a polymer network, which is thermally mendadable, was formed by the DA reaction between the furan moieties of a bisfuranic-ended polycaprolactone and the maleimide groups of a multi-maleimide compound. Polycaprolactone is a linear semicrystalline polymer, often used as a shape memory segment because of its sharp meting transition temperature range of around 40–60 °C [35]. The existence of polycaprolactone segments in the network provides the shape recovery feature under a temperature trigger, which can assist the scratch healing process via the reversible DA crosslinking reaction.
Experiment
Materials
Hexamethylene diisocyanate (99 %), 2-furfurylthiol (97 %) and triethylamine (99 %) were purchased from Sigma-Aldrich. n-Heptane (99 + %), chloroform (99 + %), tetrahydrofuran (99.5 + %) and toluene (99 + %) were purchased from Fisher Chemicals. Hexamethylene diisocyanate isocyanurate trimer (Desmodur® N 3390 BA) was received from Bayer. 3-Maleimido-1-propanol was prepared according the previously reported procedure [36]. Poly(caprolactone) diol (Capa™ 2403D, 4000 g/mol) was provided by Perstorp Chemicals Asia Pte Ltd.
Measurements
1H NMR spectra were recorded in deuterated chloroform (CDCl3) with TMS as an internal reference, on a Bruker Avance 300 at 300 MHz. Transmission Fourier transform infrared (FT-IR) spectra, collected as the average of 128 scans with a resolution of 4 cm−1, were recorded from KBr disk on the FT-IR Bruker Tensor 27. Attenuated total reflectance (ATR) FT-IR spectra were collected as the average of 128 scans with a resolution of 4 cm−1 on a FT-IR Tensor 27 spectrometer equipped with a Pike MIRacle ATR accessory with a diamond/ZnSe element. Size exclusion chromatography (SEC) measurements were performed on a Polymer PL-GPC 50 gel permeation chromatograph (GPC) system equipped with an RI detector, with chloroform as the eluent at a flow rate of 1.0 mL/min. Molecular weight and molecular weight distribution were calculated with reference to polyethylene glycol standards. Differential scanning calorimetry (DSC) measurements were carried out with a DSC Q20 V24.4 Build 116 calorimeter under nitrogen flow, at a heating rate of 10 °C/min, from −40 to 170 °C. Thermogravimetric analysis (TGA) measurements were performed under nitrogen flow using a NETZSCH STA 409 PC Instruments with a heating rate of 10 °C/min from ambient temperature to 900 °C. Tensile tests were measured at a speed of 0.05 mm/min using a Tensilon RTC-1210A tensile testing machine, making use of a 1000 N load cell. The rectangular specimens met the requirements of ASTM D882. Optical microscopic images were recorded on an Olympus GX51F microscope.
Synthesis of furyl-telechelic polycaprolactone
Hexamethylene diisocyanate (4.0 mL, 24.9 mmol), freshly azeotropically dried polycaprolactone diol (49.80 g, 12.45 mmol) and zirconium(IV) acetylacetonate (0.48 g) were dissolved in 290 mL of dry chloroform under nitrogen atmosphere. The reaction was refluxed at 65 °C under nitrogen for 4 h. After the mixture was cooled down, 2-furfurylthiol (3 mL, 29.9 mmol) and triethylamine (40 μL) were added and the mixture was stirred at room temperature under nitrogen atmosphere overnight. After the reaction, the solution was concentrated and the product was precipitated from chloroform to n-heptane for multiple times to remove the excess 2-furfurylthiol. The precipitate was dried under vacuum. Yield: 85 %.
Synthesis of the multi-maleimide crosslinker
Under nitrogen atmosphere, the mixture of Desmodur N3390 BA (2.35 g, 8.6 mmol of NCO groups), 3-maleimido-1-propanol (2 g, 12.9 mmol) and zirconium(IV) acetylacetonate (41.9 mg) in 150 mL of dry chloroform was refluxed at 65 °C for 5 h, and was then stirred at room temperature overnight. After the reaction, the solution was concentrated and the product was precipitated from chloroform to n-heptane. The product was further purified to remove excess 3-maleimido-1-propanol by thorough washing with distilled water for many times until a pure product was obtained as indicated by thin layer chromatography. Yield: 80 %.
Synthesis of the network from the bisfuranic polycaprolactone and multi-maleimide crosslinker
A mixture of bisfuranic polycaprolactone and multi-maleimide crosslinker in a 1:1 furan to maleimide equivalent ratio in tetrahydrofuran was cast in a glass petri disk at 40 °C for 48 h, followed by vacuum dried at 60 °C for 24 h.
Results and discussion
Synthesis of furyl-telechelic polycaprolactone
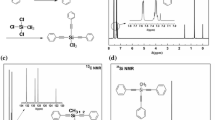
Polycaprolactone diol (Mn = 4000 g/mol; 1 equivalent) was reacted with hexamethylene diisocyanate (2 equivalents) via the alcohol-isocyanate reaction, catalyzed by zirconium(IV) acetylacetonate, to give diisocyanate terminated polycaprolactone. This intermediate product was further reacted with a slight excess of furfurylthiol by the thiol-isocyanate reaction, which was catalyzed by triethylamine [37]. The unreacted furfurylthiol was eliminated by precipitation of the resulting polymer. As a result, a polycaprolactone containing two furan moieties at the polymer chain ends was obtained. The 1H NMR spectrum of this bisfuran-terminated polycaprolactone is shown in Fig. 1, with all the peaks assigned to its chemical structure. The presence of the urethane (OCONH) peak at 4.74 ppm and thiourethane (SCONH) peak at 5.52 ppm indicates the successful occurrence of the OH-NCO and SH-NCO reactions, respectively. The presence of the furan functionality is verified by the signals at 6.24, 6.28 and 7.33 ppm. From the integration ratio between the urethane (peak u) or thiourethane (peak w) signal and the polycaprolactone units (peak m), the average molecular weight of the polymer was calculated to be 4600 g/mol. Besides, the FT-IR spectrum of the product in Fig. 2 shows typical furan absorption bands at 1011 (ring breathing and ether linkage in furan ring), 3119 and 3148 cm−1 (the double bond vibrations) [32, 38] as well as the amide (NHCO) stretch vibration at 3355 cm−1, confirming that the furan groups were attached to the polycaprolactone chains. The number-average molecular weight and molecular weight distribution of this product as determined by SEC with regard to polyethylene glycol standards were 8600 g/mol and 1.3, respectively.
Synthesis of the multi-maleimide crosslinker
The multi-maleimide crosslinker was synthesized by the alcohol-isocyanate reaction of 3-maleimido-1-propanol and the hexamethylene diisocyanate isocyanurate trimer compound bearing three isocyanate functionalities. As a result, the product containing three maleimide moieties was obtained. The 1H NMR spectrum in Fig. 3 of this product shows that all the peaks characteristic of its chemical structure can be assigned. The appearance of the maleimide peak at 6.73 ppm and the urethane peak at 4.8 ppm, as well as the slight shift of the signal of the methylene protons next to the isocyanate groups from 3.3 to 3.2 ppm, indicate the successful incorporation of the maleimide groups into the structure of the isocyanurate trimer via the urethane formation reaction. This result was confirmed by transmission FT-IR analysis. As shown in Fig. 4, the IR absorption bands at 829 and 696 cm−1 were assigned to the signals of the maleimide functionality [32].
Characterization of the network formed from the furyl-telechelic polycaprolactone and multi-maleimide crosslinker
The network was created by the DA crosslinking reaction between the furan and maleimide moieties of the furyl-telechelic polycaprolactone and multi-maleimide crosslinker, as illustrated in Scheme 1.
The occurrence of the DA reaction was evaluated by the FT-IR result. The ATR FT-IR spectrum of the obtained material in Fig. 5 shows the almost disappearance of the maleimide bands at 829, 696 and 3089 cm−1 as well as the furan bands at 1011 and 3119 cm−1, suggesting the nearly complete reaction between furan and maleimide to form DA-adduct bonds. The observation of residue maleimide bands indicates that the conversion of the DA reaction was not 100 % because of steric-hindrance effects.
ATR FT-IR spectrum of the network crosslinked from the furyl-telechelic polycaprolactone and multi-maleimide crosslinker (a), and comparisons of the spectra of the furyl-telechelic polycaprolactone (i), multi-maleimide (ii) and the obtained network (iii) in the 3440–2800 and 1035–675 cm−1 ranges (b and c, respectively)
The XRD patterns of the furyl-telechelic polycaprolactone and the cross-linked network (Fig. 6a and b) have distinct peaks at 2θ values of 21.6/21.3°, 22.1/21.9° and 23.8/23.6° similar to those of pure polycaprolactones [39], confirming the presence of the crystalline polycaprolactone phase. Both the lower 2θ values and the much lower peak intensities suggest a considerably lower degree of PCL crystallization in the network [39], as compared with the furyl-telechelic polycaprolactone. This reflects the hindered crystallization of PCL chains as a result of DA cross-links at both chain ends.
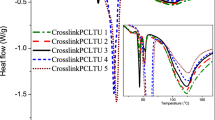
The TGA analysis of the crosslinked cast film showed that the polymer network was stable up to above 200 °C (Fig. 7), while the thermal reversibility of the DA crosslink bonds was attested by DSC analysis. Figure 8b shows that in addition to a large endotherm at around 40 °C ascribed to the melting of the crystallized polycaprolactone segments, another endothermic transition between 100 and 170 °C was observed. This transition is attributed to the breaking of the DA bonds via the retro-Diels-Alder reaction [11]. Upon subsequent cooling, two exotherms at 90–140 and 7 °C were observed and assigned to the reformation of the cleaved bonds via the Diels-Alder reaction and the re-crystallization of the PCL phase, respectively. Compared with the DSC thermogram of the furyl-telechelic polycaprolactone before cross-linking (Fig. 8a), which showed the PCL melting and crystallization peaks centered at 61 and 24 °C in the heating and cooling scans, respectively, the melting/crystallization transitions of PCL segments incorporated in the network occurred at lower temperatures and with less sharp peaks. This is because the crystallization of PCL segments were hampered by DA cross-linking netpoints, which agrees well with the XRD result.
While the thermo-reversibility of the DA bonds enables the breaking and reformation of the crosslinks, the shape-memory feature allows for the crack faces to come into intimate contact prior to the occurrence of subsequent healing reaction. For this system, the reversible melting/crystallization transition of polycaprolactone segments allows for temporary shape fixing and recovery via entropy trapping and releasing, respectively, whereas the crosslinking network via DA bonds sets the permanent shape [18]. As a result, when the material is deformed, the melting of the crystallized crosslinked polycaprolactone segments upon heating above their melting temperature results in an activation of the chain mobility, allowing them to return to their highest entropy state. Hence, the original permanent shape can be recovered.
Figure 9 demonstrates the shape-memory capability of the obtained network. The sample was deformed into a temporary spiral shape at 60 °C, which is 20 °C above the polycaprolactone melting temperature, followed by cooling to room temperature to fix this shape (Fig. 9a). By heating the sample at 60 °C, the original strip shape was fully recovered (Fig. 9b).
Assessment of the scratch healing ability
To assess the mendability of the obtained network, a scratch was made in the coating using a razor blade. Afterward, the scratch was healed by heating the scratched coating sample at 60 °C. At this temperature, the polycaprolactone segments melted, leading to an activation of the chain entropy. Thus, the material recovered its original shape via the shape memory effect. Consequently, the crack surfaces came in close proximity, so that the reversible reaction between the furan and maleimide moieties at the scratch interface could occur. On the other hand, the reformation of the broken DA bonds was favored at 60 °C [11]. The optical microscopic images in Fig. 10a shows that after 24 h at 60 °C, the scratch almost healed, with only a scar due to interface mismatch. To confirm this, another scratch was made, and was then submitted to SEM analysis. The SEM images of the scratch before and after healing shown in Fig. 10b agree well with the optical microscopic result.
Figure 11 shows that the tensile performance of the scratched film after healing at 60 °C for 2 h was still low, as compared to the initial unscratched film. Accordingly, only a maximal stress recovery of 34 % was obtained. Nevertheless, a longer heating period of 24 h led to modulus and maximal stress recovery efficiencies of slightly above 70 % (Fig. 11 and Table 1).
Conclusions
A polymer network with healing capability at mild temperature conditions were successfully prepared via the Diels-Alder reaction between a furyl-telechelic polycaprolactone and a multi-maleimide crosslinker. Remendability was possible as a result of the simultaneous occurrence of two processes at 60 °C: a shape recovery effect induced by the melting of the crystallized crosslinked polycaprolactone chains, and a progressive reformation of the DA crosslink bonds.
References
Blaiszik BJ, Kramer SLB, Olugebefola SC, Moore JS, Sottos NR, White SR (2010) Self-healing polymers and composites. Annu Rev Mater Res 40:179–211
Hillewaere XKD, Teixeira RFA, Nguyen L-TT, Ramos JA, Rahier H, Du Prez FE (2014) Autonomous self-healing of epoxy thermosets with thiol-isocyanate chemistry. Adv Funct Mater 24:5575–5583
Fereidoon A, Ghorbanzadeh Ahangari M, Jahanshahi M (2013) Effect of nanoparticles on the morphology and thermal properties of self-healing poly(urea-formaldehyde) microcapsules. J Polym Res 20:1–8
Rahimi A, Amiri S (2014) Self-healing hybrid nanocomposite coatings with encapsulated organic corrosion inhibitors. J Polym Res 22:1–8
Bergman SD, Wudl F (2008) Mendable polymers. J Mater Chem 18:41–62
Zhang Y, v Y-h, Zhang Z-p (2015) The influence of 2,4-toluene diisocyanate content on the intrinsic self-healing performance of polyurethane at room-temperature. J Polym Res 22:1–6
Cheng C, Bai X, Zhang X, Li H, Huang Q, Tu Y (2015) Self-healing polymers based on a photo-active reversible addition-fragmentation chain transfer (RAFT) agent. J Polym Res 22:1–8
Zhang MQ, Rong MZ (2013) Intrinsic self-healing of covalent polymers through bond reconnection towards strength restoration. Polym Chem 4:4878–4884
Liu Y-L, Chuo T-W (2013) Self-healing polymers based on thermally reversible Diels-Alder chemistry. Polym Chem 4:2194–2205
Mignard N, Okhay N, Jegat C, Taha M (2013) Facile elaboration of polymethylmethacrylate / polyurethane interpenetrating networks using Diels-Alder reactions. J Polym Res 20:1–13
Chen X, Dam MA, Ono K, Mal A, Shen H, Nutt SR, Sheran K, Wudl F (2002) A thermally re-mendable cross-linked polymeric material. Science 295:1698–1702
Tasdelen MA (2011) Diels-Alder “click” reactions: recent applications in polymer and material science. Polym Chem 2:2133–2145
Tian Q, Yuan YC, Rong MZ, Zhang MQ (2009) A thermally remendable epoxy resin. J Mater Chem 19:1289–1296
Zhang Y, Broekhuis AA, Picchioni F (2009) Thermally self-healing polymeric materials: the next step to recycling thermoset polymers? Macromolecules 42:1906–1912
Kavitha AA, Singha NK (2009) “Click chemistry” in tailor-made polymethacrylates bearing reactive furfuryl functionality: a new class of self-healing polymeric material. ACS Appl Mater Interfaces 1:1427–1436
Scheltjens G, Diaz MM, Brancart J, Assche GV, Mele BV (2013) A self-healing polymer network based on reversible covalent bonding. React Funct Polym 73:413–420
Bose RK, Kötteritzsch J, Garcia SJ, Hager MD, Schubert US, van der Zwaag S (2014) A rheological and spectroscopic study on the kinetics of self-healing in a single-component diels–alder copolymer and its underlying chemical reaction. J Polym Sci Part A Polym Chem 52:1669–1675
Lendlein A, Sauter T (2013) Shape-memory effect in polymers. Macromol Chem Phys 214:1175–1177
Lendlein A, Kelch S (2002) Shape-memory polymers. Angew Chem Int Ed 41:2034–2057
Lendlein A, Behl M, Hiebl B, Wischke C (2010) Shape-memory polymers as a technology platform for biomedical applications. Expert Rev Med Devices 7:357–379
Leng J, Lan X, Liu Y, Du S (2011) Shape-memory polymers and their composites: stimulus methods and applications. Prog Mater Sci 56:1077–1135
Xu H, Yu C, Wang S, Malyarchuk V, Xie T, Rogers JA (2013) Deformable, programmable, and shape-memorizing micro-optics. Adv Funct Mater 23:3299–3306
Behl M, Razzaq MY, Lendlein A (2010) Multifunctional shape-memory polymers. Adv Mater 22:3388–3410
Kirkby EL, Rule JD, Michaud VJ, Sottos NR, White SR, Månson J-AE (2008) Embedded shape-memory alloy wires for improved performance of self-healing polymers. Adv Funct Mater 18:2253–2260
Kirkby EL, Michaud VJ, Månson JAE, Sottos NR, White SR (2009) Performance of self-healing epoxy with microencapsulated healing agent and shape memory alloy wires. Polymer 50:5533–5538
Li G, Zhang P (2013) A self-healing particulate composite reinforced with strain hardened short shape memory polymer fibers. Polymer 54:5075–5086
Li G, Ajisafe O, Meng H (2013) Effect of strain hardening of shape memory polymer fibers on healing efficiency of thermosetting polymer composites. Polymer 54:920–928
Rodriguez ED, Luo X, Mather PT (2011) Linear/network poly(ε-caprolactone) blends exhibiting Shape Memory Assisted Self-Healing (SMASH). ACS Appl Mater Interfaces 3:152–161
Luo X, Mather PT (2013) Shape memory assisted self-healing coating. ACS Macro Lett 2:152–156
García-Huete N, Laza J, Cuevas J, Gonzalo B, Vilas J, León L (2014) Shape memory effect for recovering surface damages on polymer substrates. J Polym Res 21:1–10
Zhang J, Niu Y, Huang C, Xiao L, Chen Z, Yang K, Wang Y (2012) Self-healable and recyclable triple-shape PPDO-PTMEG co-network constructed through thermoreversible Diels-Alder reaction. Polym Chem 3:1390–1393
Rivero G, Nguyen L-TT, Hillewaere XKD, Du Prez FE (2014) One-Pot thermo-remendable shape memory polyurethanes. Macromolecules 47:2010–2018
Lu X, Fei G, Xia H, Zhao Y (2014) Ultrasound healable shape memory dynamic polymers. J Mater Chem A 2:16051–16060
Heo Y, Sodano HA (2014) Self-healing polyurethanes with shape recovery. Adv Funct Mater 24:5261–5268
Woodruff MA, Hutmacher DW (2010) The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog Polym Sci 35:1217–1256
Narita M, Teramoto T, Okawara M (1971) Syntheses and reactions of functional polymers. LIV. Syntheses and polymerizations of O-substituted-N-hydroxymaleimides. Bull Chem Soc Jpn 44:1084–1089
Nguyen L-TT, Gokmen MT, Du Prez FE (2013) Kinetic comparison of 13 homogeneous thiol-X reactions. Polym Chem 4:5527–5536
Mellouki A, Herman M, Demaison J, Lemoine B, Margulès L (1999) Rotational analysis of the ν7 band in furan (C4H4O). J Mol Spectrosc 198:348–357
Mani R, Bhattacharya M (2001) Properties of injection moulded blends of starch and modified biodegradable polyesters. Eur Polym J 37:515–526
Acknowledgments
This research was fully supported by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number “104.02-2013.19”.
Viet Q. Nguyen is acknowledged for the assistance with the FT-IR and GPC measurements. Tri M. Phan is acknowledged for the assistance with the tensile tests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nguyen, LT.T., Nguyen, H.T. & Truong, T.T. Thermally mendable material based on a furyl-telechelic semicrystalline polymer and a maleimide crosslinker. J Polym Res 22, 186 (2015). https://doi.org/10.1007/s10965-015-0827-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-015-0827-y