Abstract
Thermo-reversible PMMA/PU interpenetrating polymer networks were successfully prepared by Diels-Alder (DA) reaction using a furan-functionalized polymethyl methacrylate, furan-functionalized polyurethane and a maleimide-based coupling agent. First, homo-networks were synthesized and characterized in order to predict the characteristics of the corresponding interpenetrating network. Polyurethane networks were confirmed to be reversible with a temperature of retro-Diels-Alder varying from 135 °C to 144 °C depending on the cross-linking density. PMMA/PU blends are completely immiscible as shown by the presence of two Tgs corresponding to the two phases in DSC results. Due to the presence of supramolecular interactions, no phase separation was observed in the simultaneously cross-linked PMMA/PU networks. Thermal behavior and de-cross-linking of the IPNs were studied by DSC, solubility tests and rheology. Swelling tests allowed the evaluation of the networks density.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymer blends have received increasing attention because they represent an economic and efficient way to prepare new materials with attractive and variable end-use properties. Multiphase blends are generally obtained when mixing immiscible polymers owing mostly to the large size of the polymer chains that decreases the mixing entropy. They have poor mechanical performance because of the low interfacial adhesion between the polymer phases.
Polymer blending of a brittle polymer like PMMA with a rubber modifier can be an effective way to improve its physical and mechanical properties. The PMMA-based blends with thermoplastic polyurethane (TPUs) as rubber modifier are of considerable interest principally for promoting the toughness of PMMA. The interest of TPU use is due to their excellent physical properties, chemical and abrasion resistance [1].
PMMA/TPUs blend were made either in bulk [1] or in solution [2–4] from linear polymers, or synthesized in situ from monomers [5–7].
Poomalai et al. [1] prepared different PMMA/TPU blends with different proportions of TPU and with major PMMA by extrusion of both polymers. Mechanical properties and abrasive wear of different blends were studied. A significant amelioration of elongation at break was observed. This improvement is accompanied by a decrease of the tensile strength and tensile modulus. However, neat PMMA showed better wear resistance compared to PMMA/TPU blends. The cracking and deep furrows in PMMA/TPU blends observed at the material surface can explain this property.
Film polymer blends prepared by casting lead to a better interaction between the two phases as shown by Machado et al. [2–4]. By FT-IR analysis, they observed the presence of hydrogen bonds between N-H of polyurethane and C = O of PMMA from the appearance of new bands in the spectrum. They affirmed that the blend became partially miscible due to this interaction.
An alternative way of creating polymer blends is the synthesis in situ from monomers as reported by Lipatov et al. [5, 6]. The PMMA/PU blends were prepared by simultaneous polymerization of the monomers. In this case, the conversion was limited by phase separation. The blends, composed mainly of PMMA, had a typical dispersion-droplet structure. The effect of the incorporation of additives (fumed silica or metals chelates) with blend components was studied. It was reported that, especially with fumed silica, the phase separation was delayed.
One of the known methods to reduce phase separation in polymer blends is the development of IPNs. An interpenetrating polymer network is a polymer comprising two or more networks which are at least partially interlaced on a polymer scale but not covalently bonded to each other [8]. The entanglement of two cross-linked polymers leads to forced miscibility compared to usual blends and the resulting materials are expected to have a good dimensional stability [9]. Several types of IPNs can be found depending on the synthesis method: sequential IPNs, simultaneous IPNs, latex IPNs, concentration gradient IPNs, thermoplastic IPNs, semi-IPNs [10] and grafted IPNs [9].
Polyurethane/poly(methyl methacrylate) (PU/PMMA) simultaneous IPNs have been extensively studied [11–18]. One of the earliest works on PMMA/PU blend based IPN shows that the dispersion of PU particles in PMMA matrix is much thinner in an IPN than in a semi-IPN, and even more as compared to a blend of linear polymers [19].
Siddaramaiah et al. [20] reported the synthesis of PMMA/PU interpenetrating networks. The IPNs were obtained by simultaneous polymerization of castor oil, 4,4′-diphenyl methane diisocyanate, and methyl methacrylate, with benzoyl peroxide as an initiator and ethylene glycol dimethylacrylate as a cross-linker. The physico-mechanical properties, such as tensile strength, tear strength and surface hardness of PU/ PMMA IPNs increased with the increase in PMMA content. This was explained again by hydrogen bond formation between the N-H group of PU and the C = O group of PMMA. More recently, Kong et al. [21, 22] have prepared similar IPNs composed of vegetable oil based polyurethane and polymethyl methacrylate. The morphological studies showed that the IPN presents five phases: sol phase, PU-rich phase, PU-rich interphase, PMMA-rich interphase, and PMMA rich phase. When PU presents the minor proportion in the system, the authors claim that the compatibilization is improved with higher NCO/OH molar ratio.
A higher compatibilization provides superior mechanical properties compared to the equivalent blends of immiscible linear polymers. However, and because of their cross-linked structure, they are not directly re-processable.
A convenient way for maintaining the possibility of re-processability is to use thermo-reversible networks. The Diels-Alder reaction between furan and maleimide moieties and its retro are particularly attractive to form and cut chemical bonds under the effect of temperature. It is an approach that had been reported for different polymers [23–26] including PMMA [25] and PU [23–26] and for copolymers [27–33].
An organic–inorganic hybrid thermo-reversible IPN formation by means of DA reaction between maleimide and furan moieties was reported [34]. A mixture of two independent reactive systems was simultaneously cross-linked. Acid-catalyzed tetramethoxysilane was cross-linked while maleimide and furan-modified (poly(2-methyl-2-oxazoline)s were cross-linked via Diels-Alder reactions. The thermo-reversibility of IPN was obtained when the cross-linked system constitutes the major phase, via retro DA reactions.
If immiscible initial furan-functionalized polymers are used, each of them is present principally in a different phase. In case a reaction with a multiimide occurs in each phase without any interaction with the other one, two different networks should be obtained: one in each phase.
Specific interactions between the two phases (supramolecular hydrogen bond) are in favor of entanglements between the different networks that can evolve under certain conditions to an IPN. In this study, the reactive system was chosen to maximize the interactions between the two phases and to obtain an IPN. The initially immiscible polymers are PMMA and polyurethane. These two polymers were chosen because of their specific interactions that should build the networks entanglement. This original and facile method for thermo-reversible IPN’s elaboration will be evaluated in this study.
First homo-networks will be prepared. The characteristics, particularly thermal and thermo-mechanical properties of these networks will be used as reference for the characterization of the IPNs. Then IPNs will be prepared and characterized.
Experimental
Materials
4,4′-Methylenebis(cyclohexyl isocyanate) (H12MDI, mixture of isomers, 90 %), furfuryl alcohol (FAL, 98 %), dibutyltin dilaurate and different solvents (THF, ethyl acetate, DMSO and DMF) were purchased from SIGMA ALDRICH. All reagents were used without further purification.
Hydroxyl telechelic polybutadiene (HTPB) Krasol® LBH-P 3000 (Mn ~ 3,000 g.mol−1, falcohol = 1.9) was offered by Cray Valley - Hydrocarbon Specialty Chemicals.
PMMA (from BIESTERFELD PLASTIC) with a molar mass of 100,000 g.mol−1 and a glass transition of 95 °C was used.
Analysis
1H-NMR analysis
1H-NMR spectra were recorded in deuterated CDCl3 and DMSO solutions at room temperature using a Bruker Avance II spectrometer operating at a frequency of 250 MHz. The chemical shift scales were calibrated on the basis of the TMS peak (0 ppm).
Size exclusion chromatography
Size exclusion chromatography (SEC) was conducted using a system (515 Waters) equipped with a triple detector consisting of a refractive index detector (Waters 2414), a Wyatt MiniDawn Treos low angle light scattering detector and a Wyatt Visco star viscosimeter. Two columns HR 0.5 and HR 3 from Waters were used. Molar masses were determined by DDL with index of refraction increment dn/dC values calculated for each sample using Wyatt Astra 5.3.4 software. The mobile phase THF flow rate was 1 mL.min−1 and samples concentration was 3 mg.mL−1. The injection volume was 100 μL.
DSC analysis
Differential scanning calorimetry (DSC) measurements were carried out with a Q10 instrument from TA Instruments. Samples were transferred to hermetic pans that were sealed. The samples were then analyzed during two sequences of heating/ cooling between −80 °C and 200 °C at a cooling and heating rate of 10 °C.min−1. Transition temperatures (Tgs) were evaluated from the data recorded during heating by identifying the inflection points. The networks rDA temperature was determined from the first heating cycle. All the other data were collected from the second cycle.
Swelling characterization
For the swelling measurements, the gels were immersed in THF for 48 h at room temperature. Then they were removed from solvent and weighed at different times to determine the equilibrium swollen gel weight by extrapolation to the initial time. In order to obtain the weight of the dry gel, the samples were dried in a vacuum oven (2.10−1 mbar) at 25 °C for 24 h.
Rheological analysis
Rheological studies were conducted in a ARES rheometer using parallel plate geometry (25 mm). The experiments were performed within the linear viscoelastic regime under the dynamic oscillation mode using a 1 rad.s−1 frequency. The gap between plates was maintained at 2 mm. Experiments were run from 160 °C to 70 °C at a constant cooling rate of 1 °C.min−1.
Syntheses
PU networks synthesis
Furfuryl alcohol-HMM cyclo-adduct (FAL/HMM) synthesis
The used protocol of the N-hydroxymethylmaleimide (HMM) synthesis was described previously [26].
For the synthesis of FAL/HMM, 1.96 g (0.02 mol) of furfuryl alcohol and 2.54 g (0.02 mol) of HMM were refluxed for 24 h in a double necked flask under magnetic stirring. The white obtained precipitate was filtered under vacuum and washed several times with ethyl acetate. The powder was dried under vacuum for 24 h at 25 °C. The obtained yield was of 89 %.
Cross-linked polymer formation
The synthesis of networks was conducted from HTPB, H12MDI, FAL/HMM adduct and glycerol and with the dibutyltin dilaurate as catalyst. The catalyst/alcohol ratio was maintained at 10−2. Four networks PUnet1, PUnet2, PUnet3, PUnet4, with various HTPB quantity were synthesized.
As an example, the synthesis of PUnet1 was conducted as follows: 3.15 g (1.05*10−3 mol) of HTPB, 1.83 g (7*10−3 mol) H12MDI, 1.01 g (4.5*10−3 mol) FAL/HMM adduct, 0.092 g (10−3 mol) glycerol and 0.041 g (6.55*10−5 mol) of the catalyst were mixed in DMF at 80 °C for 24 h. A swollen gel was obtained. It was then dried under vacuum at 35 °C for 24 h.
PU-PMMA networks synthesis
Furan-functionalized polyurethane (PU-F) synthesis
Two PU-F, PU-F11000 and PU-F4600 with different chain lengths were synthesized from H12MDI, FAL, glycerol and HTPB. The synthesis of PU-F11000 was carried out as follows. In a double necked flask with magnetic stirring, 0.588 g (6*10−3 mol) of furfuryl alcohol was mixed with 0.184 g (2*10−3 mol) of glycerol, 6.3 g (2.1*10−3 mol) of HTPB and 0.046 g (10.1*10−5 mol) of dibutyltin dilaurate at 80 °C until solubilization. Then 2.096 g (8*10−3 mol) of H12MDI was added. The mixture was maintained at 80 °C for 3 h until complete reaction. Then the obtained solid was purified two times by dissolving in chloroform and precipitating in water. The precipitate was dried under vacuum at 35 °C for 24 h.
Furan functionalized polymethyl methacrylate (PMMA-F) synthesis
The furan-functionalized PMMA was synthesized by reactive extrusion between a commercially available PMMA and furfuryl alcohol and was described in a previous work [25].
Scheme 1 depicts the structure of obtained furan-functionalized PMMA.
Two polymers, PMMA-F13 and PMMA-F26 with furan functionalities of 13 and 26 respectively were synthesized. Their characteristics are given in Table 1.
Bis-maleimide (BMI) synthesis
The bis-maleimide synthesis was performed according to a protocol described in a previous work [25]. The obtained bis-maleimide had an average molar mass Mn = 276 g.mol−1, an average furan functionality Ffuran = 1.9 and a melting temperature Tm = 136 °C.
PMMA/PU IPN preparation
Four 75/25 (by weight) PMMA/PU blends were prepared from the different furan-functional polymers. First, the two polymers were dissolved in chloroform and maintained under magnetic stirring at room temperature for 15 min. Then, the solvent was firstly evaporated at room temperature before removal in an oven under vacuum at 35 °C for 24 h. Thin films were obtained.
Networks were prepared by mixing the previous formed blends with BMI in chloroform at room temperature under magnetic stirring by keeping the molar stoichiometry between maleimide and furyl functions. When a homogeneous solution was obtained, the solvent was evaporated and the thin film was heated in an oven at 170 °C for 15 min then cooled to room temperature at a 1 °C.min−1 cooling rate. The cross-linked blends will be noted c-blend x (where x is the number relative to blend, i.e. c-blend 1 is the blend 1 after cross-linking)
Results and discussion
A correct evaluation of the co-cross-linked PMMA/PU material should be based on the properties of both PMMA and PU homo-networks prepared by Diels-Alder reaction. The characteristics, particularly thermal and thermo-mechanical of these networks will be used as reference for the characterization of the IPNs.
The synthesis and the properties of PMMA networks equivalent to those used in this study were previously reported [25]. A preliminary study concerning PU homo-networks was realized here.
Elaboration and characterization of PU homo-networks
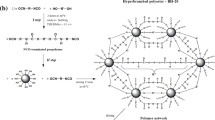
PU networks were obtained by Diels-Alder reaction in one step by reaction between a diisocyanate (H12MDI) with glycerol (cross-linking agent), two diols HTPB (macro-dialcohol monomer) and a DA adduct (thermo-reversible link) (Scheme 2).
The different quantities of reactants were calculated by maintaining constant the glycerol and the adduct concentrations. H12MDI and HTPB quantities were calculated using the Macosko-Miller equation [35] (Eq. 1) so that a cross-linking is obtained with the DA adduct formation. In this case the functionality of adduct is 2 and the pgel value must be lower than 1 (before rDA at relatively low temperature the DA adduct is bi-functional). After rDA, the adduct is dissociated and becomes mono-functional (at relatively high temperature). In this case, the calculated pgel must be higher than 1 to avoid a potential cross-linking.
With r is the alcohol/isocyanate molar ratio, ni the molar quantity of each reactant, fal and fiso respectively the average alcohol and isocyanate functionalities and fi the functionality of each alcohol.
Furfuryl alcohol-HMM (FAL/HMM) adduct synthesis and characterization
The adduct used in the network elaboration was obtained by Diels-Alder reaction between furfuryl alcohol and N-hydroxymethyl maleimide as presented in Scheme 3.
The obtained FAL/HMM adduct was first characterized by 1H-NMR (Fig. 1) to verify its purity and the obtained forms (endo and exo). As expected, and since the reaction was done at relatively high temperature, only the exo form was obtained [36]. All the peaks relative to different protons were attributed and the obtained product was pure.
The DSC was used to determine the retro-Diels-Alder temperature of the adduct. As seen in Fig. 2, the temperature associating to the rDA is about 144 °C.
-
a.
Polyurethane networks characterization
The effect of the hydroxyl-telechelic polybutadiene chain extender quantity in the network is studied here.
Solubility and swelling tests
First, solubility tests were conducted in DMSO. At room temperature, PUnet4 remains insoluble in DMSO and an important swelling was observed. When the swollen sample was heated at 160 °C, it becomes soluble (Fig. 3) as a result of a de-cross-linking. This shows clearly a thermo-reversibility of the PUnet4. Equivalent results were obtained for all the other PUnet.
Figure 4 presents the evolution of the swelling degree as a function of HTPB content. An increase of the swelling degree is observed when the HTPB content increases.
As expected, the network is denser with the minimum quantity of HTPB extender.
Thermal characterization
The DSC curves of different networks are plotted in Fig. 5. It can be noticed that two Tgs are observed. The first Tg (Tg1) represents the glass transition of the soft part composed mainly of HTPB and Tg2 is that for the hard part composed mainly of H12MDI, glycerol and DA-adduct. Also an endothermic peak is observed. It can be assimilated to the rDA phenomenon. After rDA, an exothermic peak was observed. This peak can be attributed to maleimide double bond polymerization that can lead to a secondary non-reversible cross-linking. This shows that for this system and to preserve the thermo-reversibility of cross-linking, the material should not be heated in bulk at temperatures higher than 150 °C.
The different thermal data are regrouped in Table 2. The glass transition Tg1 is almost constant for the soft part of the different networks. For TrDA, a decrease with the increase of HTPB content can be observed. Such result is expected, the network becomes looser when HTPB content increases. In this case the de-cross-linking is obtained earlier than for denser networks. The hard part Tg2 evolution is more complex. A Tg2 decrease is probably due to higher content of HTPB in this phase composed mainly of H12MDI, glycerol and DA-adduct.
Simultaneous cross-linking of PMMA/PU networks
PMMA/PU blends characterization
Obtaining a thermo-reversible cross-linked PMMA/PU blends is foreseeable by Diels-Alder reaction between furan-functionalized both PMMA and PU with bis-maleimide. As the synthesis and characterization of furan-functionalized PMMA were studied previously [25], only the synthesis and characterization of furan-functionalized PU will be studied here.
Furan-functionalized polyurethane synthesis and characterization
Furan-functionalized polyurethanes were obtained by addition reaction between a diisocyanate (H12MDI) and several alcohols (furfuryl alcohol, glycerol and HTPB). Glycerol is used as ramification agent (in order to obtain different furan functionalities), HTPB is a macro-dialcohol monomer and furfuryl alcohol is a chain limiter. The used stoichiometries were calculated while maintaining pgel > 1 using the Macosko-Miller equation to avoid a network formation.
Both prepared oligomers were analyzed by SEC (Fig. 6). The different obtained polymers present various chain lengths. The polymer PU-F4600 presents shorter chains. In fact, the presence of less quantity of HTPB allowed the obtaining of shorter chains. The average molar masses obtained by SEC are regrouped in Table 3.
Figure 7 depicts an example of 1H NMR spectrum of PU-F. Its structure can be clearly identified by the presence of different characteristic peaks. Peaks relative to the furan group (1) and (2) were, respectively at 7.41 and 6.53 ppm.
The thermal properties of the two polyurethanes are given in Table 3. The Tg of the two polymers is almost the same as it is due to the presence of HTPB in the system.
Non reactive PMMA/PU blends elaboration and characterization
In order to understand the properties and behavior of cross-linked PMMA/PU networks, a preliminary study of non reactive PMMA/PU blends is conducted.
First, thermal properties of the blends were obtained by DSC measurements. Figure 8 shows the DSC curves of the blend PMMA-F26/PU-F4600. The blend presents two different Tgs: the first one at −40 °C corresponds to the PU phase, the second one at 98 °C is relative to the PMMA phase. These Tgs are identical to those of the two polymers taken separately. This shows that these initial polymers are not miscible.
Table 4 regroups the different Tgs. For all the blends, small evolutions of Tgs are obtained probably as a result of limited specific interaction. But evidently all these blends are immiscible.
An example of rheological curves of PMMA/PU non reactive blends is given in Fig. 9. The obtained curve presents a typical of PMMA. Indeed, PMMA presents the majority phase in the blend. Two zones are identified: zone 1 which present the glass transition and zone 2 which present the rubbery zone. The rubbery zone finishes at about 160 °C where the flow begins. At this temperature most of the entanglements and hydrogen bonds are broken.
As a conclusion, immiscible PMMA/PU blends were obtained by solution mixing and rheological data show an apparent behavior of PMMA matrix. Therefore, the compatibilization of these blends by means of IPN formation will be studied in the next paragraph.
Simultaneously cross-linked PMMA/PU characterization
Solubility tests were conducted in DMSO. The example of c-blend 4 is depicted in Fig. 10. A swelling at room temperature and a solubility at 160 °C were observed. This result shows that the cross-linking of the network was successful and that the thermo-reversibility was proved. In fact, when rDA temperature is reached, the chemical bonds are broken and the initial polymers are dissolved in the DMSO. However in the case of c-blend 1 and c-blend 2, a set of pieces of cross-linked chains is observed. Such result is expected as the majority phase is PMMA and it was proven in a previous work [25] that a very loose network was obtained with PMMA-F13.
Due to swelling measurements, average molar mass between cross-links at insoluble rates can be calculated. Flory-Rehner theory is used to calculate the values of Mc between crosslinks in the interpenetrating polymeric gel. According to this theory Mc values increase with the increase of swelling ratio of gels. Molar mass between cross-links is calculated by the following equation [37].
Volume fraction of the polymer (v2) in the swollen gel is a measure of the amount of fluid that a gel can incorporate into its structure. It is calculated by the following equation [38]:
Where dp and ds are densities (g. mL−1) of the gel and solvent respectively. Ma and Mb are the weights (g) of the swollen and dry gels respectively. v2 (mL.mol−1) is the volume fraction of the swollen gel in the equilibrium state and χ is the Flory-Huggins polymer solvent interaction parameter.
Solvent interaction parameter (χ) was calculated by Flory-Huggins theory. Equation used to calculate χ value is given below [39]:
ln(1 − v 2) can be replaced in Eq. (7) by: \( \ln \left(1-{v}_2\right)=-{v}_2-\frac{v_2^2}{2}-\frac{v_2^3}{3}-\frac{v_2^4}{4}-\cdots \)
when neglecting the last terms, Eq. (7) becomes:
The swelling measurements in THF are regrouped in Table 5. It can be noticed that the insoluble rate increases with the average furan functionality. But, it is more influenced by the PMMA functionality as it presents the majority phase. In fact, an important reaction rate is observed for c-blend 3 and c-blend 4. The swelling degree was only calculated for c-blend 3 and c-blend 4 as for c-blend 1 and c-blend 2 no consistent gel was obtained. A little decrease of the swelling degree was observed when PU-F11000 was used. Indeed, the polyurethane phase presents only 25 % in the network. So, it has not a big influence in the network properties. The molar mass between cross-links is relatively high as it exceeds the molar mass of a single chain of PMMA. Probably, in addition to its role as a cross-linker, BMI allowed the grafting of polymer chains and then extending the chain length.
The DSC curves of the different networks are plotted in Fig. 11. From these curves, glass transition and retro-Diels-Alder temperatures can be determined. The different temperatures are given in Table 6. It can be noticed that only one Tg is observed varying from 51 °C to 58 °C. At first sight, no phase separation was obtained. This result can be confirmed by Fox empirical law.
Where Tg(A + B), Tg(A) and Tg(B) are respectively the glass transition temperatures of A/B miscible blend, polymer A and polymer B.
wA and wB are the weight fractions of the polymers A and B with wA + wB = 1
According to the Fox law, in the case of miscible blend of PMMA/PU, a glass transition temperature of 50 °C is obtained. So, it can be affirmed that no phase separation in the network is obtained. The obtaining of one Tg can be due to either a cross-linked copolymer PMMA-PU or an interpenetrating PMMA/PU network.
Since the two polymers present in the reactive system are initially non miscible, the cross-linking with the coupling agent begins in each phase. Then, with the favorable specific interactions, entanglements between the polymers of the two phases increase gradually leading finally to an IPN. The phase separation gradually disappeared and at the end only one Tg was obtained. It is also highly probable that coupling reactions with the bis-maleimide also occurs between PMMA and the PU. Nevertheless, it seems not realistic to consider a co-cross-linking leading to a single Tg by analogy with a random copolymer seen the high molar mass of the polymer constituents (100,000 for PMMA and for 10,000 PU).
The rDA temperature is given by the top temperature of the endothermic peak. It lies between 147 °C and 157 °C. With the increase of the average furan functionality, a decrease of the rDA temperature is observed. It can be observed that two different rDA kinetics are obtained. In fact, for blends based on PMMA-F13 a sharp peak is obtained with higher TrDA values (rapid kinetic), while for the blends based on PMMA-F26 a broad peak with lower rDA temperatures is observed (slower kinetic).
Figure 12 presents rheological curves of different networks. As the initial blend present physical interactions, the general shape of the obtained curves is identical to that obtained with the same blend without cross-linking. However, the increase of G’ before the rDA temperature and the increase of the temperature of the beginning of the flow zone show that a modification of the characteristics of the blend occurred. This modification is the result of the cross-linking. It can be also observed that the behavior of the network is almost dependent of PMMA. In fact, the glassy zone corresponds of that of PMMA. So, the PU particles act as filler in a PMMA matrix. This result confirms that an interpenetrating network was obtained where cross-linked polyurethane particles are dispersed in the polymethyl methacrylate matrix. It can be also noticed that G’ is higher for the two first networks based on PMMA-F13 than that for networks based on PMMA-F26. Denser networks are obtained for c-blend 1 and c-blend 2. This is concordant with DSC results where TrDA is higher for the two networks based on PMMA-F13.
These results are confirmed by TEM photographs where no phase separation can be observed. Therefore it can be affirmed that thermo-reversible IPNs were obtained.
Conclusions
Thermally-reversible PMMA/PU IPNs were obtained by furan/maleimide Diels-Alder reaction.
Initially non-miscible reactants conducted to mono-phasic IPNs. The de-cross-linking of the IPNs occurred between 147 °C and 157 °C depending on the reactants functionalities and the supramolecular interactions between polyurethane and PMMA.
References
Poomali, Siddaramaiah, Suresha B, Lee J-H (2008) Mechanical and three-body abrasive wear behaviour of PMMA/TPU blends. Mater Sci Eng, A 492:486–490
Patrício PSO, De Sales JA, Silva GG, Windmöller D, Machado JC (2006) Effect of blend composition on microstructure, morphology, and gas permeability in PU/PMMA blends. J Membr Sci 271:177–185
Patrício PSO, Silva GG, Machado JC (2007) Free volume properties of thermoplastic polyurethane/polymethylmethacrylate blends: evidence of interchain interaction. J Appl Polym Sci 105:641–646
De Sales JA, Patrício PSO, Machado JC, Silva GG, Windmöller D (2008) Systematic investigation of the effects of temperature and pressure on gas transport through polyurethane/poly(methylmethacrylate) phase-separated blends. J Membr Sci 310:129–140
Lipatov Y, Kosyanchuk L, Nesterov A (2002) Phase separation in blends of linear polymers formed IN SITU according to different mechanisms. Polym Int 51:772–780
Lipatov YS, Kosyanchuk LF, Yarovaya NV (2006) Effect of the interface with solid on the interfacial region in the blends of linear polymers formed in situ. J Appl Polym Sci 102:4646–4651
Shumskii VF, Kosyanchuk LF, Getmanchuk IP, Babich OV, Antonenko OI (2011) Rheology and morphology of linear polyurethane and poly(methyl methacrylate) blends formed in situ. Polym Sci Ser A+ 53:955–962
Thomas S, Boudenne A, Ibos L, Candau Y (2011) Physical, thermophysical and interfacial properties of multiphase polymer systems: state of the art, new challenges and opportunities. In: Boudenne A, Ibos L, Candau Y, Thomas S (eds) Handbook of multiphase polymer systems. Wiley, Chichester, pp 1–12
Vuillequez A, Moreau J, Garda MR, Youssef B, Saiter JM (2008) Polyurethane methacrylate/silicone interpenetrating polymer networks synthesis, thermal and mechanical properties. J Polym Res 15:89–96
Utracki LA (2002) Polymer blends handbook. Boston: Kluwer Academic Publishers, Dordrecht
Xiao HX, Frisch KC, Frisch HL (1983) Interpenetrating polymer networks from polyurethanes and methacrylate polymers. I. Effect of molecular weight of polyols and NCO/OH ratio of urethane prepolymers on properties and morphology of IPNs. J Polym Sci Polym Chem Ed 21:2547–2557
Jehl D, Widmaier JM, Meyer GC (1983) The transparency of polyurethane-poly(methyl methacrylate) interpenetrating and semi-interpenetrating polymer networks. Eur Polym J 19:597–600
Hermant I, Damyanidu M, Meyer GC (1983) Transition behaviour of polyurethane-poly(methyl methacrylate) interpenetrating polymer networks. Polymer 24:1419–1424
Jin SR, Widmaier JM, Meyer GC (1988) Kinetics of formation of polyurethane-poly(methyl methacrylate) interpenetrating polymer networks: 2. Synthesis of the rigid network in the presence of the elastomeric network. Polymer 29:346–350
Roha M, Wang B (1992) The effects of functional azo initiator on PMMA and polyurethane IPN systems. I: synthesis characterization, and thermal effects. J Appl Polym Sci 45:1367–1382
Akay M, Rollins SN (1993) Polyurethane-poly(methyl methacrylate) interpenetrating polymer networks. Polymer 34:1865–1873
Mishra V, Du Prez FE, Gosen E, Goethals EJ, Sperling LH (1995) Simultaneous interpenetrating networks of a polyurethane and poly(methyl methacrylate). I. Metastable phase diagrams. J Appl Polym Sci 58:331–346
Widmaier J, Nilly A, Chenal J, Mathis A (2005) Dependence of the phase separation process on the relative onset of network formation in simultaneous interpenetrating polymer networks. Polymer 46:3318–3322
Kim SC, Klempner D, Frisch KS, Radigan W, Frisch HL (1976) Polyurethane interpenetrating polymer networks. I. Synthesis and morphology of polyurethane-poly(methyl methacrylate) interpenetrating polymer networks. Macromolecules 9:258–263
Siddaramaiah, Mallu P, Roopa S, Somashekarappa H, Somashekar R (2005) Studies on physico-mechanical and optical properties, and WAXS of castor oil based polyurethane/polyacrylates interpenetrating polymer networks. J Appl Polym Sci 95:764–773
Kong X, Narine SS (2008) Physical properties of sequential interpenetrating polymer networks produced from canola oil-based polyurethane and poly(methyl methacrylate). Biomacromolecules 9:1424–1433
Kong X, Narine SS (2008) Sequential interpenetrating polymer networks produced from vegetable oil based polyurethane and poly(methyl methacrylate). Biomacromolecules 9:2221–2229
Marref M, Mignard N, Jegat C, Taha M, Belbachir M, Meghabar R (2013) Epoxy-amine based thermoresponsive networks designed by Diels–Alder reactions. Polym Int 62:87–98
Mallek H, Jegat C, Mignard N, Abid M, Abid S, Taha M (2013) Reversibly crosslinked self-healing PCL-based networks. J Appl Polym Sci 129(3):954–964 doi:10.1002/app.38595
Okhay N, Taha M, Mignard N, Jegat C. (2013) PMMA thermoreversible networks by Diels–Alder reaction. React Funct Polym 16(5):475–487 doi:10.1016/j.reactfunctpolym.2013.02.006
Okhay N, Mignard N, Jegat C, Taha M (2013) Diels–Alder thermoresponsive networks based on high maleimide-functionalized urethane prepolymers. Des Monomers Polym 16(5):475–487 doi:10.1080/15685551.2012.747166
Laita H, Boufi S, Gandini A (1997) The application of the Diels-Alder reaction to polymers bearing furan moieties. 1. Reactions with maleimides. Eur Polym J 33:1203–1211
Goiti E, Huglin MB, Rego JM (2003) Thermal breakdown by the retro Diels–Alder reaction of crosslinking in poly[styrene-CO-(furfuryl methacrylate)]. Macromol Rapid Commun 24:692–696
Goiti E, Huglin MB, Rego JM (2004) Some properties of networks produced by the Diels–Alder reaction between poly(styrene-co-furfuryl methacrylate) and bismaleimide. Eur Polym J 40:219–226
Kavitha AA, Singha NK (2007) Atom-transfer radical copolymerization of Furfuryl Methacrylate (FMA) and Methyl Methacrylate (MMA): a thermally-amendable copolymer. Macromol Chem Phys 208:2569–2577
Kavitha AA, Singha NK (2009) “Click chemistry” in tailor-made polymethacrylates bearing reactive furfuryl functionality: a new class of self-healing polymeric material. ACS Appl Mater Interfaces 1:1427–1436
Gaina V, Ursache O, Gaina C, Buruiana E (2012) Novel thermally-reversible epoxy-urethane networks. Des Monomers Polym 15:63–73
Gaina C, Ursache O, Gaina V, Varganici D (2013) Thermally reversible cross-linked poly(ether- urethanes). Express Polym Lett 7:636–650
Imai Y, Itoh H, Naka K, Chujo Y (2000) Thermally reversible IPN organic–inorganic polymer hybrids utilizing the Diels–Alder reaction. Macromolecules 33:4343–4346
Macosko CW, Miller DR (1976) A new derivation of average molecular weights of nonlinear polymers. Macromolecules 9:199–206
Jegat C, Mignard N (2008) Effect of the polymer matrix on the thermal behaviour of a furan-maleimide type adduct in the molten state. Polym Bull 60:799–808
Flory PJ (1953) Principles of polymer chemistry. Cornell University, Ithaca, NY
Peppas NA, Huang Y, Torres-Lugo M, Ward JH, Zhang J (2000) Physicochemical foundations and structural design of hydrogels in medecine and biology. Annu Rev Biomed Eng 2:9–29
Ranjha NM, Ayoub G, Naseem S, Ansari MT (2010) Preparation and characterization of hybrid pH-sensitive hydrogels of chitosan-co-acrylic acid for controlled release of verapamil. J Mater Sci Mater Med 21:2805–2816
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mignard, N., Okhay, N., Jegat, C. et al. Facile elaboration of polymethylmethacrylate / polyurethane interpenetrating networks using Diels-Alder reactions. J Polym Res 20, 233 (2013). https://doi.org/10.1007/s10965-013-0233-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-013-0233-2