Abstract
Poly (β-malic acid) (PMLA) could be used as a polymeric drug carrier due to its biological properties. In this paper, different definite molecular weights of PMLA for use as a polymer drug carrier were synthesized by adjusting monomer/initiator ratio in polymerization reaction. The yield of benzyl-β-malolactonate (MLABz, the major intermediate product in synthesis of PMLA) increased from the earlier reported 12 % to 32 %; and the anti-tumor drug 10-hydroxycamptothecin (HCPT) was attached to the PMLA (Mw 13 kDa) backbone through a glycine linker. The conjugation efficiency and drug release characteristics of the conjugate were determined. HCPT release from PMLA–HCPT conjugates occurred at a faster rate at an acidic pH compared with neutral pH (7.4). After 16 h of incubation at pH 5.6, 6.8 and 7.4, the released HCPT was 76.8 %, 47.2 % and 18.1 %, respectively. Human colorectal cancer SW480 cells were used to investigate the cytotoxicity of PMLA–HCPT conjugates under different pHs in vitro. The cytotoxicity of conjugate was lower than that of free HCPT in physiological pH, while it was higher in the pH 6.8 buffer solution compared to pH 7.4, due to the release of free HCPT from the PMLA–HCPT conjugates by hydrolysis. It is implied that PMLA–HCPT conjugates could be used as a promising anti-tumor polymeric prodrug.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In cancer chemotherapy, the severe drawbacks of anticancer agents limit their clinical applications [1]. Some anti-tumor drugs have poor water solubility, which causes low bioavailability and small molecular weight, making them easy to clear before they reach the action sites. Drug delivery systems that can improve drug solubility, improve pharmacokinetic properties, and deliver anticancer agents to the tumor cells, as well as controlled-release anticancer agents in vivo have been investigated [2, 3]. Some drug delivery systems based on polymers have been researched in the past decades, including polymeric micelle, dendrimer and polymer-drug conjugates, etc. [4]. Polymer-drug conjugates are the most frequent among polymer drug delivery systems, and may give new life to old active compounds abandoned due to their low solubility [5–8], as well as significantly enhance the blood circulation time of the drugs [9].
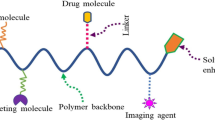
In 1975, Ringsdorf introduced the idea of tailor-made macromolecular prodrugs [10]. The drug can be attached to a water soluble or biodegradable polymer backbone by a biodegradable chemical bond or via a linker (Fig. 1). The Ringsdorf model was the basis for the development of macromolecular drug carriers.
Polymers used as drug carriers should be non-toxic, biocompatible, non-immunological, and should especially have clear reaction sites and a low polydispersity index (narrow molecular weight distribution). A lot of natural and synthetic polymers carrying multiple functional groups have been exploited for conjugation to drugs, including synthetic water-soluble polymers such as poly ethylene glycol (PEG) and poly glutamylglutamine (PGG), and natural water-soluble polymers such as hyaluronic acid (HA), heparin and albumin. Poly (β-malic acid) (PMLA) is a biocompatible, non-toxic, nonimmunogenic, and water-soluble polyester. PMLA can be degraded into malic acid, an intermediate of tricarboxylic acid cycle, and it is completely biodegraded to carbon dioxide and water [11]. It bears pendant carboxyl groups, which allow further conjugation of biologically active molecules, such as drugs, targeting and auxiliary modules [12, 13]. The carboxyl groups of PMLA provide more drug attachment sites than PEG, which has only two sites at the two ends of the polymer chain. These properties make PMLA a promising candidate carrier for anti-tumor drugs and other functional moieties [14–16]. But the difficulty involved in the preparation of PMLA limits its application. It is well known that different molecular weights of the polymer for drug delivery have different effects. For example, high molecular weight PEG with molecular weights that range from 30 kDa to 60 kDa or even higher, is used to increase the size of the nanoconjugate, leading to an increased circulation half-life and modified biodistribution profile. Low molecular weight PEG with a molecular weight less than 5 kDa, is primarily designed to increase the water solubility and oral bioavailability, or modify the biodistribution of small molecule drugs by decreasing the penetration of specific barriers [17–20].
PMLA has three kinds of structures (Fig. 2). Among of them, poly (β-malic acid) was the most widely used and had a better biocompatibility. PMLA can be produced by either biological fermentation from slime molds such as Physarum polycephalum and Aureobasidium pullulans, or by chemical synthesis starting from aspartic acid or malic acid. Currently, bio-fermentation is the main method for obtaining PMLA, but has several drawbacks [21–23]: 1) The molecular weight of PMLA is small and the range of molecular weights is difficult to control; 2) The slime mold producing PMLA is rarely and difficult to screen; and 3) The fermentation process is long and difficult to control, and the yield of PMLA is low. Direct polycondensation of malic acid invariably leads to heterogeneous polymers consisting of mixtures of α and β-malate units and molecular weights lower than 3 kDa [24]. Most poly (β-malic acid) is synthesized through the ring-opening polymerization (ROP) procedure with benzyl-β-malolactonate (MLABz) as monomer [25].
The PMLA used as a polymer carrier needs a definite molecular weight according to its specific purpose. In order to overcome the problem of side effects and to have a prolonged duration of activity, PMLA with a molecular weight less than 30 kDa has been used [14, 15]. In order to improve the targeting and pharmacokinetic properties of antibody or oligonucleotides, PMLA with a molecular weight ranging from 30 kDa to 50 kDa or higher has been used [26, 27]. In our study, different molecular weights of PMLA, ranging from 2.6 kDa to 134 kDa, were synthesized, and PMLA of Mw 13 kDa was used as 10-Hydroxycamptothecin (HCPT) carrier.
HCPT, has shown significant anti-tumor activity to colorectal, ovarian, breast, pancreas, and stomach cancers [28]. HCPT, however, like a number of other anticancer agents such as paclitaxel, has low water solubility, unique pharmacodynamics and reactivity in vivo, which have confounded its pharmaceutical development and clinical utilization. Once placed in an aqueous solution at alkaline pH, the lactone form of HCPT is quickly transformed to its carboxylate form, which has higher aqueous solubility but lower therapeutic activity [29]. For these reasons, HCPT has been conjugated to various polymeric carriers for improved solubility, enhanced stability of its lactone form and reduced renal clearance [30–33]. To adjust the rate of hydrolysis and its biological activity, HCPT could be conjugated to the polymers through different spacers, such as glycine, cysteine and alanine, etc. [30, 34]. In this study, glycine was selected as the linker between HCPT and PMLA, based on its favorable biological activity [35]. HCPT was first converted to a HCPT-amino ester (HCPT-gly), and then conjugated to the polymer via the amine bond. The transformation between the two forms of HCPT is pH dependent. There are many free carboxylic groups in the PMLA backbone; therefore the conjugate is acidic. The pH of PMLA–HCPT was 6.22 (10 mg/ml), which is conducive to the stability of the lactone form of HCPT.
Experimental
Materials
Trifluoroacetic anhydride (TFAA) was obtained from Wanxingda Chemical Co. (Jinan, China). 1-ethyl-3-(3-dimethyllaminopropyl) carbodiimide hydrochloride (EDC•HCl), triethylamine (TEA), HCPT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and Roswell Park Memorial Institute (RPMI) 1640 medium were obtained from Sigma Chemical Co. (USA). Dimethylformamide (DMF) and dimethylsulfoxide (DMSO) were obtained from Baotelai Chemicals (Xi’an, China). All solvents were thoroughly dried and distilled before use. All aqueous buffers were prepared from deionized water produced from a Milli-Q Synthesis system.
Synthesis of controlled molecular weight PMLA
PMLA was synthesized according to published routes [36], with some improvement.
Twenty-five grams (0.2 mol) of aspartic acid and 113.2 g (1.1 mol) of NaBr were dissolved in 2 mol•L−1 H2SO4. 16.6 g (0.24 mol) of NaNO2 were added to the mixture in the ice bath. The mixture was stirred for 30 min, then extracted by ethyl acetate. Organic phases were gathered and dried over MgSO4 for 1 h. After filtration, the solvent was evaporated to give product 1. One hundred grams (0.51 mol) of product 1 dissolved in anhydrous tetrohyrodfuran (THF). TFAA (0.66 mol) was added, and stirred for 2 h at room temperature; then 0.51 mol of freshly distilled benzyl alcohol was added. The mixture was stirred at 45 °C for 12 h obtain product 3, no isolation.
Benzyl-β-malolactonate (MLABz)
Two mol•L−1 NaOH was added to 5 g (12.2 mmol, 70 % of lactonizable monoester) of product 3 until the pH reached 7.5. The aqueous phase was added over 25 mL of dichloromethane at 50 °C. The mixture was vigorously stirred at 50 °C for 6 h. After decantation, the organic phase was washed until neutrality, and then dried over MgSO4. After filtration, the dichloromethane was evaporated to lead to 0.94 g of crude lactone. This lactone was purified by chromatography on silica gel (eluent: petroleum ether/dichloromethane/acetic ether, 20/20/1) and purely lactone (MLABz) was obtained, as product 4.
The influence of reaction time to MLABz yield was studied. Experimental workup was the same as described above, with different reaction times of 12 h, 24 h, and 48 h.
The influence of reaction temperature to MLABz yield was studied. Experimental workup was the same as described above under the different temperatures of 25 °C, 45 °C, 60 °C, 80 °C, and 100 °C.
PMLABz
Preparation of the initiator
Benzoic acid, 0.6 g, was added to 7.4 g 10 % (Wt%, 5 mmol) tetraethyl ammonium hydroxide in an ice bath. The resulting solution was freeze-dried to get a white solid, tetraethylammonium benzoate. The tetraethylammonium benzoate was recrystallized in a mixture of DMF/THF and dried under vacuum at room temperature. A total of 0.1 g (3.98 × 10−4 mol) of the initiator was put into 10 mL of absolute ethanol freshly distilled. Polymerization: 0.2 g (8 × 10−4 mol) of the initiator solution was placed in the polymerization flask; the solvent was eliminated under vacuum. One gram of MLABz was kept under N2 stream for 2 h and then transferred under N2 in the polymerization flask containing the initiator. The polymerization was conducted at 37 °C for 3 days (disappearance of the lactone peak at 1847 cm−l). The polymer was dissolved in acetone, one drop of concentrated HCl was added, and the polymer (product 5) was precipitated into ethanol. The PMLABz was dried under vacuum at 40 °C for 48 h.
The influence of monomer/ initiator ratio to molecular weight and distribution was studied. Experimental workup was the same as described above, with different monomer/ initiator ratios of 100/1, 500/1, 1500/1, 2000/1, and 2500/1.
The influence of temperature on molecular weight and distribution was studied. Experimental workup was the same as described above, with different temperatures of −20 °C, 0 °C, 20 °C, 37 °C, and 50 °C.
PMLA
1.0 g of PMLABz was dissolved in 20 mL 1,4-dioxane, then 0.2 g (10 %) Pd/C was added to the solution. The system was bubbled with H2 and agitated continuously for 24 h until hydrogen was no longer reduce. The Pd/C was filtered off and washed with 1,4-dioxane, the merged filtrate was concentrated by solvent evaporation and the concentrated solution was dropped into ethyl ether to give the de-protected polymer of PMLA; a white solid, product 6. The PMLA precipitates were dried under vacuum at room temperature for 2 h. The whole synthetic scheme is shown in Fig. 3.
All intermediate products and the final product structure were determined by Fournier transform infrared spectroscopy (FT-IR) using KBr disc (Shimadzu FTIR-8400S) and proton nuclear magnetic resonance (1H NMR) using Varian 400 mHz (Bruker, Avance). The sample for 1H NMR was prepared by dissolving in DMSO-d6 (10 mg•mL−1) at 25 °C. Chemical shifts were given in units relative to the tetramethyl silane (TMS) signal as an internal reference. The molecular weight of PMLA was determined by gel permeation chromatography (GPC) in regard to polystyrene using THF as mobile phase at flow rate of 0.5 mL•min−1.
Synthesis of PMLA–HCPT conjugates
t-Boc-glycine (377 mg, 2.1 mmol) was dissolved in 200 mL of anhydrous dichloromethane, then HCPT (263 mg, 0.72 mmol), dicyclohexylcarbodiimide (DCC) (444 mg, 2.1 mmol) and 4-dimethylaminopyridine (DMAP) (87 mg, 0.72 mmol) were added to this solution at 0 °C. The reaction mixture was stirred for 1 h and subsequently allowed to reach room temperature, and was left overnight. The solution was washed with 0.1 mol L−1 HCl, dried and evaporated under reduced pressure to yield a white solid, which was recrystallized from methanol to give hydroxycamptothecin-10‑ester of t-Boc-glycine, The t-Boc protection group was removed by dissolving in a mixture of CH2Cl2 (15 mL) and TFA (trifluoroacetic acid) (15 mL), stirred at room temperature for 1 h. Solvent was removed and the solid was recrystallized from CH2Cl2 and ether to give hydroxycamptothecin-10-glycinate, HCPT-gly.
PMLA (11.6 mg, 0.1 mmol monomer) and HCPT-gly (42.1 mg, 0.1 mmol) were dissolved in 10 mL of anhydrous dimethyl sulfoxide (DMSO); EDC•HCl (19.2 mg, 0.1 mmol) and triethylamine (TEA) (14 μL, 0.1 mmol) were added to this solution at room temperature under N2 atmosphere. The reaction mixture was stirred for 1 h and subsequently allowed to reach room temperature and left overnight. The product was dialyzed using molecular weight cutoff 2,000 membranes in DMSO for 2 days to remove excess reagents and unconjugated HCPT. Then the product was further dialyzed against millipore water for 2 days to remove DMSO. The resulting solution was freeze-dried to obtain a yellow powder. The whole synthetic scheme is shown in Fig. 4.
The intermediate products and the final product structure were determined by FT-IR using KBr disc (Shimadzu FTIR-8400S) and 1H NMR using Varian 400 mHz (Bruker, Avance). Solubility of PMLA–HCPT was determined by dissolving the conjugate in deionized water at 25 °C and HCPT grafting percent was determined by UV spectrophotometer at 413 nm. The grafting percent of HCPT was calculated as following:
Release of HCPT from the conjugate
In vitro drug release profiles were obtained by a dynamic dialysis method [33]. The release experiments were conducted at 37 °C. Typically, 20 mg of PMLA–HCPT conjugate was placed into a dialysis bag and introduced to 200 mL of phosphate-buffered solution (0.1 M PBS, pH 5.6, 6.8, 7.4,respectively) with magnetically stirring at 200 rpm. At hourly intervals, 0.5 mL samples were removed from the release medium and PBS at the same volume and temperature was added to the release medium.
The released HCPT was determined by high performance liquid chromatography (HPLC) (Diamonsil C18 column: 5 μm, 200 mm × 4.5 mm; C18 pre-column: 5 μm, of 10 mm × 4.6 mm; mobile phase: methanol/acetonitrile/water (20:18:62); fluorescence detector detection: excitation wavelength 363 nm, emission wavelength of 550 nm; flow rate: 1.0 mL • min−1; injection volume: 20 μL; sensitivity: AUFS = 1.0; column temperature: 25 °C). Results of triplicate tests data were used to calculate accumulated drug release.
Measurement of cytotoxic activity against SW480 colorectal cancer cell lines
Cell culture
Human colorectal cancer SW480 cells (kindly provided by the Department of Pharmacology, School of Pharmacy Xi’an, China) were employed as in vitro models. The cells were cultured in RPMI 1640 medium containing 10 % heat-activated fetal bovine serum and 100 IU/mL penicillin and 100 g/mL streptomycin. They were incubated in a 37 °C water-jacketed incubator equilibrated with 5 % CO2 and kept at approximately 99 % relative humidity. The medium was replenished every other day until confluence was achieved. The cells were then washed with PBS and harvested with 0.125 % Trypsin–EDTA solution.
In vitro cytotoxicity
Human colorectal cancer SW480 cells were seeded at a density of 1 × 104 cells/well in 96-well transparent plate and incubated for 24 h. All growth medium was prepared by supplementing DMEM with 5 % penicillin–streptomycin, 10 % fetal bovine serum, and sterilized with 0.22 μm filter prior to use. The medium was then replaced by PMLA, the free HCPT, or PMLA–HCPT conjugate at various drug concentrations from 0.01 to 1 mg/mL in the medium. These were then incubated for another 48 h before replacing the medium within each well with 0.1 mL of fresh growth medium and 20 μL of MTT solution. After incubation for another 4 h, 0.15 mL of DMSO was added to each well to dissolve any formazan crystals that had formed. The plates were vigorously shaken before measuring the relative color intensity using a microplate reader at 490 nm. Cell viability for that particular concentration of sample was expressed as a percentage of the intensity of the controls ± standard deviation. Each experiment was repeated five times at each polymer concentration. The SW480 cell viability of PMLA was also investigated at various concentrations equivalent to those for the conjugate.
Results and discussion
Preparation of MLABz and synthesis of controlled molecular weight PMLA
Preparation of MLABz
The synthesis of MLABz was the key step in preparation of PMLA. The yield of MLABz was lower than 12 % according to Reference [36]. In this paper, we studied the influence of reaction time and temperature on the yield of MLABz. With the extension of the reaction time from 6 h to 24 h (Table 1), the yield of MLABz was improved. The influence of reaction temperature was investigated (Table 2). At room temperature, this intramolecular esterification reaction could not be easily performed. But if the temperature was higher than 80 °C, the product of lactone was not stable. So, 50 °C should be an appropriate temperature for this reaction. For this reaction, when the temperature was 50 °C, the reaction time was 24 h, the yield raised from the ealier reported 12 % to 32 %.
Synthesis of controlled molecular weight PMLA
Chemical synthesis methods were able to obtain PMLA of controlled molecular weight, but the low yield of reaction limits the applications. The molecular weight of a polymer is an important factor in a polymer–drug delivery system: low molecular weight polymer–drug conjugates exhibit a nonspecific biodistribution profile, short plasma circulation time and rapid systemic elimination; while too high of a molecular weight may influence the water solubility and reaction activity of conjugates. For PMLA as a drug carrier, a molecular weight between 4 kDa and 50 kDa is appropriate. PMLA of different molecular weights has different applications as a polymeric drug carrier. The molecular weight of PMLA in transporting doxorubicin was 4 kDa [37, 38]. The molecular weight of PMLA in plycefin ranges from 30 kDa to 50 kDa or higher, in order to prolong circulation time for brain tumor targeting [39, 40]. In this work, different molecular weights of PMLA, ranging from 2.6 kDa to 134 kDa,were synthesized by controlling the ratio of monomer/ initiator. PMLA of Mw 13 kDa was used as HCPT carrier, taking into account the properties of HCPT.
The number average molecular weight (Mn) and weight average molecular weight (Mw) and the molecular weight distribution (Mw/Mn) of polymer samples were determined by GPC relative to polystyrene equipped with triple detectors. Tetraethylammonium benzoate was used as initiator in the ring-opening polymerization of MLABz. The influence to molecular weight and distribution by the monomer/ initiator ratio was analyzed. As shown in Fig. 5, the Mn and Mw of PMLA increased with the growth of the monomer/ initiator ratio. According to different purposes, the controlled molecular weight of PMLA could be attained. The influence of temperature on molecular weight and distribution was investigated in this work; as can be seen in Table 3, with increase of temperature, the molecular weight of products decreased slightly. In order to get the desired molecular weight polymers, 50 °C or room temperature was used most in our follow-up works.
Characterization of PMLA and PMLA–HCPT conjugates
PMLA has been successfully synthesized, by 1H-NMR and IR. Characteristics of PMLA: 1H-NMR (400 MHz, DMSO-d6, 6 ppm): 3.02 (m, 2H); 5.11 (s, 2H); 5.41 (m, 1H). IR (v, cm−1): 1743 (C = O), 3421 (OH).
The synthetic scheme of PMLA–HCPT conjugate is shown in Fig. 4. In the present study, a two-step synthetic strategy was adopted to covalently link HCPT onto PMLA backbone. HCPT was first modified with boc-gly to obtain HCPT-gly. HCPT was chemically conjugated to PMLA via primary amide bonds using EDC•HCl, NHS and TEA as coupling agents in dimethylsulfoxide (DMSO) to produce PMLA–HCPT conjugate.
1H NMR in DMSO-d6 was used to confirm the product identity. 1H NMR spectrum of HCPT-gly showed no peaks in the hydrox region (9.8 ppm); these were present in the original HCPT (Fig. 6).
The HCPT content in the PMLA–HCPT conjugate was determined by the UV spectrophotometer with UV detection at 413 nm. Attachment of HCPT to the PMLA carrier via amide bond resulted in a more hydrophilic water-soluble polymer, enabling higher loading of HCPT up to 14.38 wt.%.
As far as the solubility was concerned, PMLA–HCPT conjugates in this study exhibited good solubility in water. For instance, 16.71 ± 0.06 mg of the freeze-dried PMLA–HCPT was easily dissolved in 1 mL of deionized water, in which the effective concentration of HCPT would be 2.4 mg•mL−1. These data were higher than the water solubility of HCPT, whose solubility in deionized water is 0.0025 mg•mL−1. So it is possible to infer that these polymer–drug conjugates could prolong the circulation time and the mean residence time. The in vivo pharmacokinetics profile of the PMLA–HCPT conjugate is under study.
In vitro release of HCPT from the conjugate
In vitro release of the drug from PMLA–HCPT conjugate was measured at various pH conditions. As shown in Fig. 7, the HCPT release showed no dramatic initial burst in PMLA–HCPT conjugate. The lower the pH value, the faster the drug was released. Specifically, the drug released rapidly from the PMLA–HCPT conjugate at pH 5.6, reaching 76.8 % after 16 h, whereas the HCPT release at pH 7.4 was much slower, 18.1 % in the same period, respectively (p < 0.05). A possible mechanism is that the linker of glycine between PMLA and HCPT hydrolyzes with the prolongation of time, making the acidity of the medium increase and then speeding up the hydrolysis of amide bond. Thereby, the rate of drug release would accelerate. By this polymer design, the conjugates can preserve drugs under physiological conditions and selectively degrade and release them by responding the tumor extracellular pH (pHe), endosomes (pH 5–6) or lysosomes (pH 4–5) [41, 42].
In vitro cytotoxicity
Human colorectal cancer SW480 cells were used to investigate the cytotoxicity of PMLA–HCPT conjugates under different pHs compared to the free HCPT as a positive control. The relative cytotoxicity of PMLA–HCPT conjugate and the pure drug are shown in Figs. 8 and 9. Figure 8 showed the cell viability of PMLA–HCPT, HCPT and PMLA at pH 7.4. These results suggested that the cytotoxicity of PMLA–HCPT conjugates against SW480 cells were lower than free HCPT under physiological pH, which means PMLA–HCPT conjugate had lower toxicity to normal tissues and cells in vivo cycle. Figure 9 shows the cell viability of PMLA–HCPT, HCPT and PMLA at pH 6.8. It is obvious that the cell viability decreased with increase of the drug concentration. The relative cytotoxicity of PMLA–HCPT was higher at pH 6.8 compared to the physiological pH, on account of the release of free HCPT from the PMLA–HCPT conjugates. At pH 5.6, cellular activity of the comparative group was as low as 20 %, and that of the treatment group was even lower (< 10 %) (data not shown). It is inferred that at this pH, the acidic environment had an influence on cell viability that was greater than drugs.
Conclusion
To obtain a controlled molecular weight of PMLA, the ring-opening polymerization process of MLABz (the key intermediate in synthesizing of PMLA), and especially the influence of monomer/ initiator ratio and temperature on the values of molecular weights, was studied. With aspartic acid as a starting material, different molecular weights of PMLA, ranging from 2.6 kDa to 134 kDa, were synthesized through the ring-opening polymerization procedure with MLABz as monomer. The yield of MLABz was raised from the early reported 12 % to 32 %. HCPT was conjugated to PMLA and a novel PMLA–HCPT conjugate was obtained. Compared to free HCPT, the PMLA–HCPT conjugate showed lower cytotoxicity under physiological pH, which means low toxicity to normal tissues and cells during in vivo circulation. Meanwhile, HCPT could be released from the conjugate by hydrolysis at tumor extracellular pH (pHe), endosomes (pH 5–6) or lysosomes (pH 4–5), to show anti-tumor activity. This implies that PMLA–HCPT conjugates could be a promising HCPT carrier for intracellular delivery.
References
Pasut G, Veronese FM (2009) Adv Drug Deliv Rev 61:1177
Duncan R, Gac-Breton S, Keane R, Musila R, Sat YN, Satchi R, Searle F (2001) J Control Release 74:135
Lopez-Davila V, Seifalian AM, Loizidou M (2012) Curr Opin Pharmacol 12:414
Felber AE, Dufresne MH, Leroux JC (2012) Adv Drug Deliv Rev 64:979
Khandare J, Minko T (2006) Prog Polym Sci 31:359
Fan L, Wu H, Zhang H, Li F, Yang T (2009) Polym Compos 31:51
Fan L, Li F, Zhang H, Wang Y, Cheng C, Li X, Gu CH, Yang Q, Wu H, Zhang S (2010) Biomaterials 31:5634
Canal F, Sanchis J, Vicent MJ (2011) Curr Opin Biotechnol 22:894
Bamrungsap S, Zhao Z, Chen T, Wang L, Li C, Fu T, Tan W (2012) Nanomedicine 7:1253
Ringsdorf H (1975) J Polym Sci Polym Symp 51:135
Ljubimova JY, Fujita M, Ljubimov AV, Torchilin VP, Black KL, Holler E (2008) Nanomedicine 3:247
Braud C, Bunel C, Vert M (1985) Polym Bull 13:293
Inoue S, Ding H, Portilla-Arias J, Hu J, Konda B, Fujita M, Espinoza A, Suhane S, Riley M, Gates M, Patil R, Penichet ML, Ljubimov AV, Black KL, Holler E, Ljubimova JY (2011) Cancer Res 71:1454
Ouchi T, Kobayashi H, Banba T (1990) Br Polym J 23:221
Ohya Y, Hirai K, Ouchi T (1992) Makromol Chem 193:1881
Huang ZW, Laurent V, Chetouani G, Ljubimova JY, Holler E, Benvegnu T, Loyer P, Cammas-Marion S (2012) Int J Pharm 423:84
Pendri A, Gilbert CW, Soundararajan S, Bolikal D, Shorr RGL, Greenwald RB (1996) J Bioact Compat Polym 11:122
Greenwald RB, Pendri A, Bolikal D, Gilbert CW (1994) Bioorg Med Chem Lett 4:2465
Greenwald RB, Gilbert CW, Pendri A, Conover CD, Xia J, Martinez A (1996) J Med Chem 39(424)
Yamaoka T, Tabata Y, Ikada Y (1994) J Pharm Sci 83:601
Manitchotpisit P, Skory CD, Peterson SW, Price NP, Vermillion KE, Leathers TD (2012) J Ind Microbiol Biotechnol 39:125
Leathers TD, Manitchotpisit P (2013) Biotechnol Lett 35:83
Qiao CS, Zhong K, Hao HX, Jia YY (2012) J Appl Biomater Biom 108:121
Kajiyama T, Kobayashi H, Taguchi T, Kataoka K, Tanaka J (2004) Biomacromolecules 5:169
Coulembier O, Degée P, Hedrick JL, Dubois P (2006) Prog Polym Sci 31:723
Ljubimova JY, Fujita M, Khazenzon NM, Lee BS, Wachsmann-Hogiu S, Farkas DL, Black KL, Holler E (2008) Chem Biol Interact 171:195
Fujita M, Lee B, Khazenzon NM, Penichel ML, Wawrowsky KA, Patil R, Ding H, Black KL, Ljubimova JY (2007) J Control Release 122:356
Li QY, Zu YG, Shi RZ, Yao LP (2006) Curr Med Chem 13:2021
Mi Z, Burke TG (1994) Biochemistry-Us 33:10325
Hatefi A, Amsden B (2002) Pharm Res 19:1389
Li Q, Liu C, Zhao X, Zu Y, Wang Y, Zhang B, Zhao D, Zhao Q, Su L, Gao Y, Sun B (2011) Int J Nanomedicine 6:397
Minko T, Paranjpe PV, Qiu B, Lalloo A, Won R, Stein S, Sinko PJ (2002) Cancer Chemother Pharmacol 50:143
Cho J, Chun C, Kuh H, Song S (2012) Eur J Pharm Biopharm 81:582
Dharap SS, Qiu B, Williams GC, Sinko P, Steind S, Minko T (2003) J Control Release 91:61
Greenwald RB (2001) J Control Release 74:159
Cammas S, Renard I, Langlois VR, Guerin P (1996) Polymer 37:4215
Cao N, Feng S (2008) Biomaterials 29:3856
Abdellaoui K, Bousttaa M, Vert M, Morjanib H, Manfaitb M (1998) Eur J Pharm Sci 6:61
Ding H, Inoue S, Ljubimov AV, Patil R, Portilla-Arias J, Hu J, Konda B, Wawrowsky KA, Manabu Fujitab NK, Takako Sasakie KLB, Holler E, Ljubimova JY (2010) PNAS 107:18143
Portilla-Arias J, Patil R, Hu J, Ding H, Black KL, Garcia-Alvarez M, Munoz-Guerra S, Ljubimova JY, Holler E (2010) J Nanotechnol. doi:10.1155/2010/825363
Wojtkowiak JW, Verduzco D, Schramm KJ, Gillies RJ (2011) Mol Pharm 8:2032
Tian L, Bae YH (2012) Colloid Surface B 99:116
Acknowledgments
This study was supported by National Nature Science Foundation of China grants No. 81271687 and No. 30970788.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Youbei Qiao and Xiao Duan contributed equally to this work.
Rights and permissions
About this article
Cite this article
Qiao, Y., Duan, X., Fan, L. et al. Synthesis of controlled molecular weight poly (β-malic acid) and conjugation with HCPT as a polymeric drug carrier. J Polym Res 21, 397 (2014). https://doi.org/10.1007/s10965-014-0397-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-014-0397-4