Abstract
Directly starting from lactic acid (LA) and trimesic acid (TMA), novel biodegradable material poly(lactic acid-trimesic acid) (PLT), a modified polylactic acid (PLA) with terminal carboxyl, was synthesized via melt copolycondensation. The optimal synthetic conditions, including catalyst kinds and dosage, prepolymerization time, copolymerization temperature and time, were discussed. When L-lactic acid (L-LA) and TMA as molar feed ratio n(L-LA)/n(TMA) 120/1 was prepolymerized for 8 h at 140 °C, the copolycondensation catalyzed by 0.9 wt % SnCl2 at 190 °C for 8 h gave PLT with the biggest intrinsic viscosity ([η]) 1.91 dL∙g−1, and the corresponding weight-average molecular weight (Mw) was 14,100 Da. Serial L-PLTs at different molar feed ratios were synthesized and characterized with FTIR, 1H NMR, GPC, DSC, and XRD. Increasing n(L-LA), Mw increased first, and the biggest Mw was 17500 Da at n(L-LA)/ n(TMA) 240/1, then decreased. Using D,L-lactic acid (D,L-LA) instead of L-LA, the influences of LA stereochemical configuration were investigated. The peak phenomenon of Mw was similar, but the biggest Mw was 23,100 Da at n(D,L-LA)/n(TMA) 320/1. The serial L-PLTs had a certain crystallinity (10.2%∼23.0%), while all D,L-PLTs were amorphous. These differences may be in touch with the reaction mechanism of direct melt copolycondensation. The method was simple and practical for the synthesis of PLA biomedical materials applied in drug delivery carrier, and vessel substitution material.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For its excellent biocompatibility, biodegradability and bioabsorbability, polylactic acid (PLA) has been widely applied in biomedical fields, including surgical suture, bone fixation material, drug delivery carrier, and tissue engineering scaffold materials [1–5]. However, the familiar PLA was linear, and the linear polylactic acid (LPLA) has some drawbacks in properties, including lower thermal stability at high temperature and higher glass transition temperature (Tg), which limited its more possible applications. Thus, the star-shaped polylactic acid (SPLA) that could offset some drawbacks of LPLA, is being highlighted [6, 7].
In the published papers, multifunctional hydroxyl compounds were usually used as the core in the synthesis of SPLA [6–13]. And there are few reports on the synthesis of SPLA using multifunctional carboxyl compounds as the core. This may be related to the limitation of preparation methods for SPLA. In most cases, SPLA was synthesized via traditional ring-opening polymerization (ROP) of lactide usually using hydroxyl compounds as initiator [7]. Compared with hydroxyl group, organic acid could accelerate the ROP of lactide, but the activity of carboxyl group is too low to initiate the ROP of lactide [14–16].
On the other hand, when SPLA was synthesized via the direct melt copolycondensation of lactic acid (LA) with other multi-functional carboxyl compounds, there is only a few papers reported, and the limited researches showed that the molecular weight of product is lower. For example, when directly starting from L-lactic acid (L-LA) and citric acid, the biggest weight-average molecular weight (Mw) of the copolymer synthesized via direct melt polycondensation was only 5378 Da, and polydispersity index (PDI) Mw/Mn was 2.96 [17–19]. When directly starting from D,L-lactic acid (D,L-LA) and sebacic acid, the biggest Mw of the copolymer synthesized via direct melt copolycondensation was 15,500 Da, but in most cases Mw was less than 9,700 Da and the biggest PDI (Mw/Mn) was 2.18 [20, 21].
However, the synthesis of PLA copolymer using carboxyl compounds as starting materials can make the surface of PLA biomaterials have more acidic groups, which is beneficial for the PLA materials with pendant carboxyl to improve their cell affinity and control the speed of materials degradation [19, 22], or to be a hopeful material for vessel substitution with good blood compatibility [23]. Therefore, Wang’s group prepared the functionalized biodegradable copolymer poly(L-lactide-co-malic acid) via a longer route with five steps including the preparation of different cyclic intermediates, the ROP of L-lactide and the deprotection of benzyl [22, 24].
In fact, even only for the preparation of lactide, which has usually been prepared from LA, or the derivative of LA, the synthetic process was also lengthy and troublesome, and the yield was low. At the same time, to synthesize higher molecular weight PLAs, the intermediate lactide must be recrystallized several times before they are used; this process is repetitious and consumes a lot of organic solvents [1]. Thus, how to use a simple method to obtain PLA material (e.g. SPLA) with acidic surface or terminal carboxyl group is worth exploring.
In recent years, there are more and more reports on the direct syntheses of PLA [25–27] or its copolymers [28, 29] using LA as starting material, especially via melt homo- [30–40] /co- [41–50] polycondensation of LA, the novel simple one-step method instead of the traditional two-step method using lactide as an intermediate. However, there is no report on the synthesis of SPLA via direct melt copolycondensation using trimesic acid (TMA) as the core. At the same time, only in a few papers [28, 39, 51–53] the mechanism of direct melt polycondensation was discussed, even the mechanism of direct melt copolycondensation was seldom mentioned before.
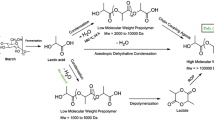
In this paper, based on our previous work [7], especially the researches on the direct melt homo-/co- polycondensation of LA, and the application in drug delivery [54–59], we respectively used L-LA and D,L-LA as starting materials, investigated their copolycondensation with TMA (Scheme 1). A novel SPLA with terminal carboxyl group, copolymer poly(lactic acid-trimesic acid) (PLT) was successfully synthesized and systematically characterized by FTIR, 1H NMR, GPC, DSC and XRD. Based on the analysis of the influences of different molar feed ratio and LA stereochemical configuration, for the first time the peak phenomena of molecular weight were reported when increasing the molar feed ratio n(LA)/ n(TMA), and the possible mechanism of direct melt copolycondensation was proposed.
Experimental
Materials
L-LA was purchased from Wako Pure Chemical Industries, Ltd. (Tokyo). TMA was purchased from Alfa Aesar China (Tianjin) Co., Ltd. Other chemical reagents, including stannous chloride (analytic reagent), D,L-LA (analytic reagent), were purchased from Guangzhou Chemical Reagent Factory (Guangzhou, China). All these materials were used without further purification.
Instrumental analysis and measurements
1H NMR spectra were recorded with a Varian NMR system 400 MHz (USA) with CDCl3 as the solvent and TMS as internal standard. IR spectra were obtained from an FTIR spectrometer (Bruker Vector 33, Ettlingen, Germany) by the KBr salt slice method.
The intrinsic viscosity ([η]) of PLT was determined with Ubbelohde viscometer (Cannon-Ubbelohde, State College, PA) using CHCl3 as solvent at 25 °C. According to literatures [60–62], the relative molecular weight and molecular weight distribution of the polymer were determined by gel permeation chromatography (Waters 1515 pump, Torrance, CA) with tetrahydrofuran as solvent at 35 °C and a flow velocity 1 mL∙min−1. Three Styragel HR columns from Japan covering a molecular weight range of 1 × 103–106 Da were used and calibrated using five polystyrene narrow standards from BF Goodrich (Richfield, Ohio). Molecular weight distributions for the samples were calculated using the Millennium 2010 software from Waters and were reported as polystyrene equivalent values. As a comparative reference, the relative molecular weight was also tested via the titration method [51, 63].
DSC was performed with Perkin-Elmer DSC7 thermal analyzer (Perkin-Elmer, Cetus Instruments, Norwalk, CT) at a heating rate of 10 °C∙min−1 under a nitrogen atmosphere (flow velocity, 20 mL∙min−1) and the transition temperature was calculated from the second run. With a wavelength of 1.5406 × 10−10 m and a scanning scope of 2θ from 1° ∼ 40° with Cu Kα radiation, a Rigaku D/max-2000X X-ray diffractometer (Dandong, China) was used to investigate the crystallinity of PLT.
Prepolymerization
According to the previous work on melt homo-/co- polymerization of LA [54–59], LA and TMA should be prepolymerized before copolymerization. After LA and TMA were uniformly mixed as preplanned molar feed ratio, the mixture was directly dehydrated for 4–12 h at 140 °C under 4,000 Pa in a flask equipped with mechanical stirring and thermometer.
Melt copolymerization
After prepolymerization, the selected catalyst was added in according to the weight percent (wt %) of dehydrated reactants. The melt copolymerization was carried out at a certain temperature (140–200 °C) and an absolute pressure of 70 Pa for 4–12 h. When the reaction finished, the purification via the dissolution in CHCl3 and the subsequent precipitation by CH3OH ordinarily produced a white powder after drying in vacuo.
Results and discussion
Recently, increasing importance has been attached to the direct melt homo-/co- polycondensation of LA for its simple process, high efficiency and low cost [30–50]. Thus, in this paper, the novel biodegradable material PLT was synthesized via direct melt polymerization. And in most cases, L-LA has been used more extensively than D,L-LA. Therefore, selecting L-LA as the starting material, the optimal synthetic conditions for the synthesis of PLT were first investigated.
Optimal synthetic conditions
The influences of catalyst kinds and dosage, prepolymerization time, copolycondensation temperature and time on the synthesis of PLT were investigated. The results in Table 1 showed that the effect of catalyst was in touch with metal kinds. In terms of molecular weight of the copolymer, though the appearance of all products was white powder and the catalyst SnO where the biggest yield (61%) for PLT was observed, SnCl2 was the best catalyst among the five for its biggest [η] (Run 2).
The influences of catalyst SnCl2 quantity on [η] of PLT are shown as Table 2. When the quantity of SnCl2 was 0.9 wt %, [η] reached a maximum (Run 5), too much or less was not appropriate. When the catalyst dosage was too small, the reaction was so insufficient after a certain time that [η] was not big. When the quantity of SnCl2 was excessive, short-chain molecule was apt to be formed through the degradation of polymer, which also was catalyzed by SnCl2, so [η] was not big too.
The influences of prepolymerization time on [η] of PLT are shown as Table 3. It was obvious that [η] reached a maximum after the reaction lasted for 8 h (Run 3). When the time was too shorter, prepolymerization was insufficient. However, once the reaction time was longer than 8 h, the oxidation and thermal degradation of polymer became serious. So, [η] dropped. Thus, the appropriate prepolymerization time should be 8 h.
When the melt copolycondensation was carried out respectively at different temperatures and the results are shown as Table 4. The higher reaction temperature was advantageous for the melt copolycondensation. However, when the temperature was 200 °C, [η] markedly decreased. At the same time, the yield was the lowest of all, and the appearance of product was not white as other cases, for the side reactions such as oxidation and thermal degradation markedly took place at 200 °C. So, the appropriate temperature was 190 °C (Run 6).
The influences of melt copolycondensation time on [η] of PLT are shown as Table 5. It was obvious that [η] reached a maximum after the reaction lasted for 8 h (Run 3). Therefore, when the molar feed ratio n(L-LA)/n(TMA) was 120/1, the optimal synthetic conditions for PLT were as follows: prepolymerization time 8 h, catalyst 0.9 wt % SnCl2, and the melt copolycondensation at 190 °C for 8 h. Under these conditions, the biggest [η] was 1.91 dL∙g−1, and GPC determination showed that the corresponding Mw was 14,100 Da.
Under the above optimal synthetic conditions, serial L-PLTs at different molar feed ratio were synthesized with the yield of 23% ∼ 40% (Table 6).
Structure characterization of PLT
The structure of PLT was characterized with FTIR. Though the molar feed ratio n(LA)/n(TMA) was different, the IR data of the serial PLTs were almost same. As a representative, the data of PLT synthesized at molar feed ratio n(L-LA)/n(TMA) 120/1 were listed as follows. IR (KBr, cm−1): 3444 (terminal COOH); 2996 and 1408 (saturated C–H, including CH3 and CH); 1760 (C=O); 1629, 1560 and 1459 (benzene ring skeleton); 1186, 1133 and 1091 (C–O–C in ester group); 871 (1,3,5-trisubstituted benzene ring).
The structure of PLT was also characterized with 1H NMR (CDCl3 as solvent and TMS as internal standard). Similarly, the chemical shift data of PLT with different molar feed ratio n(LA)/n(TMA) were almost same. Using PLT synthesized at molar feed ratio n(L-LA)/n(TMA) 120/1 as a representative (Fig. 1), the data of 1H NMR were showed as follows. 1H NMR (δ, ppm): 1.52 (CH3 in LA chain segment, H d ), 4.38 (CH in terminal LA unit), 5.18 (CH in LA chain segment, H c ), 5.79 (terminal COOH, H b , a low and broad peak for the exchange of active hydrogen with the solvent CDCl3), 8.95 (Ar–H in TMA segment [64], H a ).
Therefore, the structure of PLT was basically demonstrated by FTIR and 1H NMR as expected (Scheme 1(1)). However, the following analysis of PLT molecular weight showed that in some cases the structure of PLT was not a simple star-shaped polymer that only embedded a TMA core as Scheme 1(1).
Influences of different molar feed ratio on L-PLT molecular weight
It was also shown in Table 6 that the influences of different molar feed ratio on PLT molecular weight. Obviously, the biggest [η] was 2.35 dL∙g−1 at n(L-LA)/n(TMA) 240/1, and the corresponding Mw determined by GPC was 17,500 Da, the biggest Mw of all with the biggest PDI 3.07 also (Run 4). Of course, most of PDI was more than 2.0. However, there are many literatures on PLA materials with PDI 2.0 ∼ 4.5, involving in the different synthetic methods, such as the ROP of lactide [9, 14, 22, 65–67], and the direct melt polymerization of LA [17, 21]. In our experiments, the phenomenon of the bigger PDI may be related to the formation of multi-core structure in the copolymerization.
At the same time, most of Mw was more than 10,000 Da, and the smallest was 7,100 Da (Table 6, Run 1). Usually, when the PLA polymers were used as drug delivery material, their molecular weights were no more than 30,000 Da [55, 68]. As reported in the literatures, the PLA materials with molecular weight of 1,800 Da could be applied in drug delivery, even the PLA polymers with molecular weight of only 900 Da could be used as drug delivery carrier [41, 69]. Therefore, the molecular weight of PLT synthesized here via direct melt copolycondensation meets the requirement for drug delivery application at the least.
In Table 6, MNMR (the molecular weight calculated from 1H NMR measurement, based on the integral of H d and H a ) in most cases was less than the molecular weight as theoretical molar feed ratio for the escape of LA out of the reaction systems as lactide during the melt copolycondensation (in fact, this is also the reason why the yield of PLT usually was low), and the change regularity of MNMR was not in consistent with that of number-average molecular weight (Mn) determined by GPC. In order to look for more reference, titration method [51, 63], a chemical analysis method, was also used to test the molecular weight of PLT for the existence of its terminal carbonyl as Scheme 1(1). And the calculated results of molecular weight (MT) were also showed in Table 6.
Obviously, MT had greater difference from the results from GPC, it was markedly lower than the corresponding Mn in most cases, and only MT at molar feed ratio n(LA)/n(TMA) 640/1 was closer each other (Run 7). This may be related to the calculation method of MT. The formula of MT was based on the premise that PLT was a simple star-shaped polymer as Scheme 1(1), and one polymeric molecule only had three carboxyl groups. Thus, once a molecule had more than three carboxyl groups, the factual MT would be bigger than the corresponding calculated MT in Table 6.
Therefore, supposing that some terminal carboxyl groups were changed into anhydride groups (Scheme 1(2), an anhydride group was equivalent to two carboxyl group in the titration), a relatively reasonable explanation may be given out as the following. Except for n(LA)/n(TMA) 640/1, there were multi-core phenomena in other cases. In other words, not only one TMA core was embedded in the polymer (Scheme 1(2) and (3)). Referring to the corresponding Mn of the serial PLTs, the approximate number of TMA core may be 5, 3, 5, 4, 2, 2 and 1, respectively, with the increase of n(LA) in Table 6. The formation of multi-core structure and its change regularity of basically gradual reduction may be related to the mechanism of the direct melt copolycondensation, which will be specially discussed in the later.
Influences of different molar feed ratio on thermal properties and crystallinity of L-PLT
The serial L-PLTs were characterized with DSC, and the results were listed in Table 7. Compared with the linear poly(L-lactic acid) (LPLLA, Tg 50.0 °C, Tm 134.1 °C [56]) synthesized via the direct melt polycondensation, the L-PLTs data of Tg were lower in most cases, which showed that the introduction of the core TMA lowered the Tg in some degree. But the data of Tm were higher in all cases, which showed that the introduction of the core TMA had a certain effect on thermal properties. However, the melting heat (ΔH) of L-PLT was obviously lower than that of LPLLA (46.7 J∙g−1 [56]), indicating that the crystallinity of the star-shaped polymer was decreased for the introduction of TMA, and this conclusion was further confirmed by the results of XRD characterization (Table 8).
The crystallinity of polymers has an important effect on their physical and biological properties, especially their degradability which is crucial for biomaterials. The results showed that the position of the XRD absorption peak of all L-PLTs was basically the same as the position of LPLLA (using PLT synthesized at molar feed ratio n(L-LA)/n(TMA) 120/1 as a representative, Fig. 2). However, compared with the crystallinity of LPLLA (45.1% [56]), the crystallinity of serial L-PLTs was markedly lowered (Table 8) because of the introduction of TMA as the core. For the same reason, the crystallite size of L-PLTs markedly became bigger than that of LPLLA (D110 143.4 × 10−10 m, D020 69.5 × 10−10 m [56]).
Influences of different LA stereochemical configuration on PLT
Using D,L-LA instead of L-LA, we also synthesized serial D,L-PLTs under the same synthetic conditions. Though the data of D,L-PLT structure characterization by FTIR (using PLT synthesized at molar feed ratio n(D,L-LA)/n(TMA) 320/1 as a representative, Fig. 3) and 1H NMR were almost similar with those of L-PLT, the other influences of different LA stereochemical configuration on PLT were different, and shown as Table 9.
The GPC results showed that the biggest Mw was 23,100 Da at n(D,L-LA)/n(TMA) 320/1 with the biggest PDI 3.21 also (Table 9, Run 5), even bigger than the biggest Mw of L-PLT. It is well known that D,L-LA is more inexpensive than L-LA. Thus, starting from D,L-LA and TMA, the direct melt copolycondensation may provide a low-cost way to synthesize the PLA materials with higher molecular weight.
Similarly, based on the analyses of the molecular weights tested by different methods (Table 9), it could be found that there were multi-core phenomena still. With the increase of n(D,L-LA), the approximate number of TMA core in the serial D,L-PLT may be 5, 5, 2, 2, 4, 4 and 2, respectively. At the same time, the change regularity that the core number in D,L-PLT gradually decreased was basically similar with that of L-PLT also. However, the molar feed ratio at the biggest Mw was different. Therefore, though different LA starting material could be copolymerized via the similar reaction mechanism, a little difference resulted from the different LA stereochemical configuration may be existed still.
The serial D,L-PLTs were tested by DSC and XRD, and the corresponding data were listed in Table 9 (neither the melting peak on DSC curve nor the absorption peak on XRD spectra was detected, so there was no data on Tm, ΔH or crystallinity). Compared with linear poly(D,L-lactic acid) (LPDLLA, Tg 54.6 °C, Tm 120.0 °C and crystallinity 20.8% by XRD method) synthesized via the direct melt polycondensation [56], the introduction of TMA not only affected the thermal properties of D,L-PLT (Tg was obviously lowered), but also altered D,L-PLT’s crystallinity to be completely amorphous. However, all serial L-PLTs were partially crystalline still (Tables 7 and 8). These indicated that the LA stereochemical configuration had a greater effect on the crystallinity of polymer, which may be connected with the mechanism of the direct melt copolycondensation also.
Possible mechanism of direct melt copolycondensation of LA and TMA
The direct melt copolycondensation of LA with TMA was the dehydration condensation via the esterification of carboxyl (–COOH) and hydroxyl (–OH) [28, 39, 51–53]. TMA molecule containing three carboxyl groups can react with the hydroxyl of LA molecule to form three polyester (PLA) arms containing terminal carboxyl each one. Then, when n(LA) was big enough, the continuing esterification of arms with other LA gave a star-shaped polymer with one TMA as the core (Scheme 1(1)). In this case, the Mn of GPC was basically in line with MT, that of the titration (Table 6, Run 7).
However, this was only the main reaction; the side reaction of the formation for the anhydride structure also via the dehydration between different PLA arms was inevitable because of the terminal carboxyl (Scheme 1(2)). Once two or more polymeric molecules were connected each other, the molecular weight of the product would be drastically increased, at least be doubled. Thus, even at the molar feed ratio n(L-LA)/ n(TMA) 640/1, the PDI was so big (Mw/Mn 2.60, Table 6, Run 7).
Similarly, because of the competition between the normal esterification and the inevitable formation of anhydride under the same reaction conditions, once the molar feed ratio n(LA)/n(TMA) was not big enough, the multi-core structure may be apt to emerge as the form of macromolecular anhydride. And the more TMA, the more chance for producing anhydride, therefore the more cores embedded in (Scheme 1(3)). Of course, the anhydride also could take part in the esterification with the hydroxyl of LA (Scheme 1(4)). So, in other words, increasing n(LA) could reduce the core number, which is indeed as the experiments showed, no matter what the LA stereochemical configuration is.
Obviously, the molecular weight of the product was simultaneously dependent on two factors, the core number and the arm length. With the increase of n(LA), the approximate core number was gradually reduced, but the arm length was becoming longer (Scheme 1(4)). Therefore, the increase of Mw was not a simple linear growth. The biggest Mw was related to the multi-core structure and bigger PDI, and there was a peak phenomenon of Mw. This conclusion will be helpful for the synthesis of PLA biodegradable materials via the direct melt copolycondensation of LA with multifunctional compounds.
Due to the different reactivity and reaction characteristic of LA [56], L-LA was more apt to yield the longer L-PLA arms with better tacticity than D,L-LA in the main reaction (Scheme 1(1)), while the shorter D,L-PLA arms produced from D,L-LA was more apt to be amorphous. The quick elongation of L-PLA arms was not advantageous for the formation of anhydride (Scheme 1(2)), but was further beneficial to the formation of the polymer with better tactility. Therefore, compared with D,L-PLT, the multi-core structure of L-PLT disappeared faster when n(LA) was big enough (Scheme 1(4)). At the same time, L-PLT kept partial crystallinity still, while D,L-PLT was amorphous for the more multi-core structure and the worse stereoregularity of D,L-PLA arms.
Conclusion
Directly using TMA and LA as starting monomers, PLT was synthesized via melt copolycondensation. The novel PLA copolymer with terminal carboxyl group or acidic surface may be an important potential biodegradable material applied in the biomedical fields, including drug delivery carrier, and vessel substitution material [19, 22, 23]. The biggest Mw of L-PLT was 17,500 Da, far bigger than that of the reported polymer synthesized via the direct melt copolycondensation of LA with other multifunctional organic acid, e.g. citric acid [17–19] and sebacic acid [20, 21]. Even using cheaper D,L-LA instead of L-LA, the biggest Mw of D,L-PLT was 23,100 Da.
The synthetic method is practical for its simpler process, higher efficiency and lower cost. At the same time, the direct melt copolycondensation of LA is very suitable for the starting material containing carboxyl, such as TMA, which could not be directly utilized in the ROP of lactide for the reactivity of carboxyl group [14–16]. More importantly, based on the analyses of the influences of different molar feed ratios and LA stereochemical configuration, the peak phenomena of Mw were found for the first time. These explorations may be valuable for the synthesis of more PLA biodegradable materials via the direct melt copolycondensation of LA with other multifunctional compounds.
References
Kricheldorf HR (2001) Chemosphere 43:49
Mehta R, Kumar V, Bhunia H, Upadhyay SN (2005) J Macromol Sci Part C Polym Rev 45:325
Liu MX, Dong J, Yang YJ, Yang XL, Xu HB (2008) J Nanosci Nanotechno 8:3493
Kumbar SG, Nukavarapu SP, James R, Nair LS, Laurencin CT (2008) Biomaterials 29:4100
Lim LT, Auras R, Rubino M (2008) Prog Polym Sci 33:820
Hao QH, Li FX, Li QB, Li Y, Jia L, Yang J, Fang Q, Cao A (2005) Biomacromol 6:2236
Luo YF, Wang ZY, Song XM, Mao ZZ (2008) Prog Chem 20:1578
Danko M, Libiszowski J, Biela T, Wolszczak M, Duda A (2005) J Polym Sci Part A Polym Chem 43:4586
Tsuji H, Miyase T, Tezuka Y, Saha SK (2005) Biomacromol 6:244
Yu X, Tang XZ, Pan CY (2005) Polymer 46:11149
Yuan WZ, Yuan JY, Zheng SX, Hong XY (2007) Polymer 48:2585
Gottschalk C, Wolf F, Frey H (2007) Macromol Chem Phys 208:1657
Sobczak M, Witkowska E, Olędzka E, Kolodziejski W (2008) Molecules 13:96
Gottschalk C, Frey H (2006) Macromol 39:1719
Fu HL, Zou T, Cheng SX, Zhang XZ, Zhuo RX (2007) J Tissue Eng Regen Med 1:368
Zou T, Cheng SX, Zhang XZ, Zhuo RX (2007) J Biomed Mater Res B Appl Biomater 82:400
Yao FL, Bai Y, Zhou YT, Liu C, Wang H, Yao KD (2003) J Polym Sci Part A Polym Chem 41:2073
Yao FL, Chen W, Liu C, Yao KD (2003) J Appl Polym Sci 89:3850
Yao FL, Bai Y, Chen W, An XY, Yao KD (2004) Eur Polym J 40:1895
Modi S, Jain JP, Kumar N (2005) Isr J Chem 45:401
Modi S, Jain JP, Domb AJ, Kumar N (2006) Eur J Pharm Biopharm 64:277
He B, Bei JZ, Wang SG (2003) Polymer 44:989
Wang W, Liu Y, Wang J, Jia XH, Wang L, Yuan Z, Tang SM, Liu M, Tang H, Yu YT (2009) Tissue Eng A 15:65
He B, Wan YQ, Bei JZ, Wang SG (2004) Biomaterials 25:5239
Ajioka M, Enomoto K, Yamaguchi A (1995) Bull Chem Soc Jpn 68:2125
Yoda S, Bratton D, Howdle SM (2004) Polymer 45:7839
Lei ZQ, Wang SF, Bai YB (2007) J Appl Polym Sci 105:3597
Ajioka M, Suizu H, Higuchi C, Kashima T (1998) Polym Degrad Stab 59:137
Lassalle V, Galland GB, Ferreira ML (2008) Bioproc Biosyst Eng 31:499
Moon SI, Lee CW, Miyamoto M, Kimura Y (2000) J Polym Sci Part A Polym Chem 38:1673
Moon SI, Lee CW, Taniguchi I, Miyamoto M, Kimura Y (2001) Polymer 42:5059
Moon SI, Kimura Y (2003) Polym Int 52:299
Qian G, Zhou XG, Zhu LB, Yuan WK (2003) J Polym Eng 23:413
Chen GX, Kim HS, Kim ES, Yoon JS (2006) Eur Polym J 42:468
Takasu A, Narukawa Y, Hirabayashi T (2006) J Polym Sci Part A Polym Chem 44:5247
Nagahata R, Sano D, Suzuki H, Takeuchi K (2007) Macromol Rapid Comm 28:437
Jahno VD, Ribeiro GB, Dos Santos LA, Ligabue R, Einloft S, Ferreira MRW, Bombonato-Prado KF (2007) J Biomed Mater Res A 83:209
Iwahashi H, Oka T, Abiko A (2008) Chem Lett 37:708
Wang WC, Wu LB, Huang Y, Li BG (2008) Polym Int 57:872
Pandey A, Aswath PB (2009) J Biomater Sci Polym Ed 20:33
Wang N, Wu XS, Lujan-Upton H, Donahue E, Siddiqui A (1997) J Biomater Sci Polym Ed 8:905
Gao QW, Lan P, Shao HL, Hu XC (2002) Polym J 34:786
Moon SI, Deguchi K, Miyamoto M, Kimura Y (2004) Polym Int 53:254
Lan P, Zhang YP, Gao QW, Shao HL, Hu XC (2004) J Appl Polym Sci 92:2163
Duan JF, Du J, Zheng YB (2007) J Appl Polym Sci 103:3585
Olewnik E, Czerwinski W, Nowaczyk J, Sepulchre MO, Tessier M, Salhi S, Fradet A (2007) Eur Polym J 43:1009
Liu CB, Jia WJ, Qian ZY, Huang MJ, Gu YC, Chao GT, Gou ML, Gong CY, Deng HX, Lei K, Huang AL, Tu MJ (2007) J Polym Res 14:31
Du J, Fang YY, Zheng YB (2007) Polymer 48:5541
Tsuji H, Matsuoka H, Itsuno S (2008) J Appl Polym Sci 110:3954
Su JY, Chen YW, Tan LC (2009) J Biomater Sci Polym Ed 20:99
Hiltunen K, Seppälä JV, Harkonen M (1997) Macromol 30:373
Osaka I, Watanabe M, Takama M, Murakami M, Arakawa R (2006) J Mass Spectrom 41:1369
Harshe YM, Storti G, Morbidelli M, Gelosa S, Moscatelli D (2007) Macromol React Eng 1:611
Zhao YM, Wang ZY, Wang J, Mai HZ, Yan B, Yang F (2004) J Appl Polym Sci 91:2143
Zhao YM, Wang ZY, Yang F (2005) J Appl Polym Sci 97:195
Wang ZY, Zhao YM, Wang F, Wang J (2006) J Appl Polym Sci 99:244
Wang ZY, Zhao YM, Wang F (2006) J Appl Polym Sci 102:577
Wang ZY, Hou XN, Mao ZZ, Ye RR, Mo YQ, Finlow DE (2008) Iran Polym J 17:791
Wang ZY, Li XW, Li JN, Li GM, Tao JQ (2009) J Polym Res 16:255
Arvanitoyannis I, Nakayama A, Kawasaki N, Yamamoto N (1995) Polymer 36:2947
Zhang WA, Zheng SX (2007) Polym Bull 58:767
Gou PF, Zhu WP, Shen ZQ (2008) J Polym Sci Part A Polym Chem 46:2108
Tuominen J, Kylma J, Seppälä JV (2002) Polymer 43:3
Ge J, Yan M, Lu DN, Zhang ML, Liu Z (2007) Biochem Eng J 36:93
Zhu KJ, Lin XZ, Yang SL (1990) J Appl Polym Sci 39:1
Jabbari E, He XZ (2008) J Mater Sci Mater Med 19:311
Lou L, Yin JB, Gao ZT, Liang QZ, Dong LS, Chen XS, Jing XB (2003) Polym Mater Sci Eng 19:72
Zhou SB, Deng XM, Li XH, Jia WX, Liu L (2004) J Appl Polym Sci 91:1848
Wang N, Wu XS (1998) J Biomater Sci Polym Ed 9:75
Liu HJ, Hsieh CT, Hu DSG (1994) Polym Bull 32:463
Acknowledgements
We are grateful to the financial support by Guangdong Provincial Natural Science Foundation of China (No. 5300082) and National Natural Science Foundation of China (No. 20772035). We also thank Dr & Prof. Ling-Ting YANG for helpful discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, ZY., Luo, YF., Ye, RR. et al. Synthesis of novel biodegradable material poly(lactic acid-trimesic acid) via direct melt copolycondensation and its characterization. J Polym Res 18, 499–508 (2011). https://doi.org/10.1007/s10965-010-9442-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-010-9442-0