Abstract
Fully biodegradable poly(l-lactide)/poly(triethylene glycol adipate) blends with different phase behaviors and significantly increased crystallization rate were prepared for the first time in this research. Depending on PTGA content, PLLA/PTGA blends showed different phase behaviors. PLLA was completely miscible with PTGA when PTGA content was not higher than 15 mass%, while PLLA was partially miscible with PTGA when PTGA content was higher than 15 mass%. Increasing the PTGA content remarkably increased both the overall crystallization rate and spherulitic growth rate of PLLA, evidencing the plasticization role of PTGA; however, irrespective of the presence of PTGA, both the crystallization mechanism and the crystal structure of PLLA remained unchanged.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Poly(l-lactide) (PLLA) has been widely used in many practical fields as a biodegradable aliphatic polyester [1, 2]. The physical properties of PLLA significantly depends on the crystallinity developed during the processing. For instance, the heat deflection temperature of amorphous PLLA is only about 60 °C, around its glass transition temperature; however, it can be remarkably increased if a high crystallinity of PLLA is achieved. The biodegradation rate of PLLA is also significantly influenced by the crystallinity, because amorphous region is easier to degrade than the crystalline region. In addition, the mechanical, optical, and barrier properties of PLLA are also greatly affected by the crystallinity. Therefore, it is of great interest and importance to study the crystallization behavior of PLLA. The crystallization rate of PLLA is usually very slow, depending on the detailed crystallization conditions, such as cooling rate and crystallization temperature. If the cooling rate is faster than 20 °C/min, the melt crystallization process of PLLA cannot be detected; therefore, amorphous PLLA may be obtained. In addition, the crystallization rate also depends on the D-lactide composition of PLLA. With increasing the d-lactide composition, the crystallization rate of PLLA decreases.
The following two methods are often used to accelerate the crystallization of PLLA. One is the use of heterogeneous nucleation agent, which decreases the nucleation activation energy barrier, makes the nucleation easier at a high crystallization temperature, and thus enhances the crystallization rate of PLLA. Until now, some efficient nucleation agents, such as poly(lactide) stereocomplex, poly(glycolic acid), cellulose nanocrystals, orotic acid, cyanuric acid, carbon nanotubes, polyhedral oligomeric silsesquioxanes, zinc phenylphosphonate, and graphene oxide, have been extensively studied [3,4,5,6,7,8,9,10,11]. The other is the use of plasticizer, which increases the spherulitic growth rate as well as the overall crystallization rate of PLLA by reducing the glass transition temperature and improving the chain mobility. Until now, some plasticizers, such as poly(ethylene glycol) (PEG), poly(ethylene adipate) (PEA), and poly(diethylene glycol adipate) (PDEGA) have been reported to be efficient for the crystallization of PLLA [12,13,14,15,16,17].
Poly(triethylene glycol adipate) (PTGA) shows the similar chemical structure as PDEGA; therefore, PTGA may also behave as a novel plasticizer for the crystallization of PLLA. To our knowledge, the effect of PTGA on the crystallization of PLLA has not been reported in literature so far. In this research, the miscibility and crystallization behavior of PLLA/PTGA blends were investigated with various techniques in detail. The novelties of this work may be described as follows. On the one hand, fully biodegradable PLLA/PTGA blends were prepared for the first time; moreover, PTGA was found to be a novel plasticizer to enhance the crystallization of PLLA. On the other hand, depending on the mass ratio of PTGA, PLLA/PTGA blends displayed different phase behaviors, i.e., the homogeneous phase in miscible polymer blend and two-phase structure in partially miscible blend. This research is expected to be of significant importance and interest in both biodegradable polymer blends and polymer crystallization.
Experimental
Materials and preparation of PLLA/PTGA blends
PLLA (4032D, 98% l-lactide, Tg = 60 °C, and Tm = 165 °C) was purchased from Nature Works, which had an Mn of 1.90 × 105 g mol−1 and a PDI of 1.63.
PTGA was synthesized through a two-stage polycondensation method in our laboratory, which had an Mn of 1.0 × 103 g mol−1 and a PDI of 2.35 [18].
Using chloroform as a cosolvent, PLLA/PTGA blends with different mass ratios of 90/10, 85/15, 80/20, and 70/30 were prepared through a typical solution and casting process. For comparison, neat PLLA was treated through the same process.
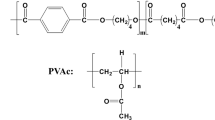
Scheme 1 displays the chemical structures of PLLA and PTGA.
Characterizations
The characterizations were similar to those described elsewhere [15], which are shown in the supplementary materials for brevity.
Results and discussion
Phase behavior study of PLLA/PTGA blends
Phase behavior study is of significant importance in polymer blends. In this research, the phase behavior of PLLA/PTGA blends was first investigated by differential scanning calorimetry (DSC) and scanning electron microscopy (SEM). Figure S1 in the supplementary materials displays the DSC curves for the PLLA, PTGA, and PLLA/PTGA blends during the quenching process at 60 °C min−1. Both PLLA and PLLA/PTGA blends did not crystallize due to the slow crystallization rate of PLLA; therefore, all samples reached the completely amorphous state. Figure 1 shows the DSC traces of the two neat components and their blends at a heating rate of 20 °C min−1 after a melt-quenching process. For the two neat components, PLLA showed a glass transition temperature (Tg) at 60.0 ℃ while PTGA displayed a Tg at − 56.8 ℃. The two neat components showed a significant difference in Tg (about 120 °C), which would favor the obvious decrease in the Tg of PLLA component induced by PTGA as a plasticizer in the blends. For the blends, one or two Tg values were observed depending on the blend composition, showing different phase behaviors. In the case of 90/10 and 85/15 samples, only one single Tg was detected at 38.1 and 36.0 °C, respectively, which were intermediate between those of the two neat components, revealing that PLLA and PTGA were completely miscible in the amorphous region. In the case of 80/20 and 70/30 samples, two composition-dependent Tg values were found, corresponding to those of the two components, respectively. For instance, an 80/20 blend showed two Tg values at 33.5 and − 55.2 °C, corresponding to those of PLLA phase and PTGA phase, respectively, while they became 30.4 and − 55.5 °C in the case of a 70/30 blend. The phase behavior in the 80/20 and 70/30 samples evidenced that PLLA and PTGA should be partially miscible in the amorphous region. In brief, PLLA/PTGA blends showed different phase behaviors depending on the blend composition. With the increase of PTGA composition, the variation from miscible one phase to partially miscible two phases was found for PLLA/PTGA blends. In addition, the Tg of PLLA phase significantly decreased to 30.4 °C in a 70/30 blend, suggesting that PTGA should be an efficient plasticizer and would effectively affect the crystallization behavior of PLLA.
In addition, it was interesting to compare the effectiveness of PTGA to some other plasticizers. For instance, the ΔTg/plasticizer content was 21.9 K 10%−1 for PTGA in this work, while in the case of PEG, PEA, and PDEGA, the values were about 17, 13.6, and 15.5 K 10%−1, respectively [12, 15, 16], indicating that PTGA was an efficient plasticizer.
SEM was further used to directly observe the phase morphology of PLLA/PTGA blends. The fractured surfaces of neat PLLA and PLLA/PTGA blends were first etched by tetrahydrofuran (THF) for 30 min and then observed with SEM. Figure 2a, b display that one homogeneous phase was found for neat PLLA and the completely miscible blend (such as 90/10). However, Fig. 2c demonstrates that two-phase morphology was clearly observed for the partially miscible PLLA/PTGA blend (such as 80/20), as PTGA phase was soluble and etched by THF, showing a large number of small dark holes on the surface of PLLA phase. In brief, the phase morphology study by SEM was consistent with the DSC results.
Isothermal melt crystallization kinetics study
The physical properties of semicrystalline polymers are related to the crystallinity formed during the polymer processing. Figure 3 depicts the crystallization time dependence of relative crystallinity of neat PLLA and 80/20 at indicated crystallization temperature (Tc) values. For each sample, it required longer time to complete the crystallization with increasing Tc, suggesting slower crystallization rate. In addition, the blend needed shorter crystallization time than neat PLLA at the same Tc, indicating that PTGA accelerated the crystallization of PLLA due to its effective plasticizer role.
We further analyzed the isothermal melt crystallization kinetics of neat PLLA and PLLA/PTGA blends with the well-known Avrami equation:
where Xt is the relative crystallinity at crystallization time (t) [19, 20]. Figure 4 displays the Avrami plots of neat PLLA and 80/20, demonstrating four nearly parallel straight lines; consequently, the Avrami equation could accurately describe the isothermal melt crystallization kinetics of neat PLLA and 80/20. The Avrami parameters n and k of neat PLLA and PLLA/PTGA blends are listed in Table 1. According to the Avrami equation, n is the Avrami exponent reflecting the nucleation mechanism and growth geometry of the crystals, while k is the crystallization rate constant including both nucleation and crystal growth processes [19, 20]. In this work, the n values of all samples were between 2 and 3, manifesting that neat and blended PLLA crystallized via the same crystallization mechanism. The k values gradually decreased with increasing Tc for each sample, indicating a slower crystallization rate; moreover, the k values obviously increased with the PTGA content, suggesting a faster crystallization rate. Crystallization half-time (t0.5) was further applied to compare the overall crystallization rate of neat PLLA and PLLA/PTGA blends. Through the following equation, t0.5 was calculated.
To better understand the influence of PTGA content on the crystallization rate of PLLA in the blends, Fig. 5 demonstrates the plots of t0.5 versus degree of supercooling (ΔT) for neat PLLA and PLLA/PTGA blends. On the one hand, t0.5 of each sample gradually decreased with increasing ΔT, suggesting a faster crystallization rate due to the greater crystallization driving force. On the other hand, t0.5 obviously varied for different PTGA contents at the same ΔT; moreover, the temperature at which the maximum crystallization rate occurred should also vary for different PTGA contents. The reason would be discussed later in the spherulitic morphology and growth study by polarized optical microscopy (POM).
Spherulitic morphology and growth rate study
The spherulitic morphology and growth rate were investigated with POM for neat PLLA and PLLA/PTGA blends. Figure 6 depicts the POM images for neat PLLA and PLLA/PTGA blends after they finished crystallizing at 120 ℃ and filled the whole volume. As clearly shown in Fig. 6, with an increase in PTGA content, the size of PLLA spherulites gradually became larger; consequently, the number of PLLA spherulites remarkably decreased. The spherulitic morphology variation suggested that the nucleation density of PLLA spherulites decreased after the blending with PTGA. Such variation is common in miscible or partially miscible crystalline/amorphous polymer blends, as the amorphous plasticizer plays a diluent role in the crystallization of the crystalline component [12,13,14,15,16,17].
In addition, the spherulitic growth rate (G) values were further measured in a wide Tc range for neat PLLA and PLLA/PTGA blends. Figure 7 displays the variation of G with Tc for all samples. In the case of neat PLLA, the G values first increased, reached a maximum, and then decreased with an increase in Tc in the investigated Tc range, showing a very common bell shape in polymer crystallization. In the case of PLLA/PTGA blends, they showed the similar bell shapes to that of neat PLLA, regardless of the blend composition. At the same Tc, the blends showed greater G values than neat PLLA; moreover, the higher the PTGA content, the greater the G value. The increase in G in the blend should mainly be related to the decreased Tg of PLLA phase. From a polymer crystallization viewpoint, the decreased Tg favored the mobility of polymer chain and should be helpful for the polymer to move and arrange into ordered structure, thereby enhancing the spherulitic growth rate.
Crystal structure study
Figure 8 presents the wide angle X-ray diffraction (WAXD) profiles of all samples, after they finished crystallizing at 125 ℃ for 9 h. PLLA shows polymorphism depending on crystallization conditions; moreover, the most thermally stable α-form will be developed when PLLA is crystallized from the melt at and above 120 °C [21]. In the present study, neat PLLA was crystallized at 125 °C; therefore, it should display the α-form crystal structure. From Fig. 8, the two typical diffraction peaks at 2θ = 16.8° and 19.2° were attributed to the (110)/(200) and (203) planes, respectively, of the α-form of PLLA crystals [21]. In addition, regardless of PTGA content, all the four PLLA/PTGA blend samples displayed the similar diffraction WAXD patterns as neat PLLA. Consequently, amorphous PTGA did not modify the crystal structure of PLLA.
Conclusions
To accelerate the crystallization and extend the application, PLLA/PTGA blends with different mass ratios were prepared for the first time in this research with PTGA being a novel plasticizer. Depending on PTGA content, PLLA/PTGA blends showed different phase behaviors. When PTGA content was not higher than 15 mass%, only one single glass transition temperature and one phase morphology were found, indicating that PLLA was completely miscible with PTGA. When PTGA content was higher than 15 mass%, two glass transition temperatures, which varied with PTGA content, and two-phase morphology were observed, suggesting that PLLA was partially miscible with PTGA. Both the overall crystallization rate and spherulitic growth rate of PLLA remarkably increased with increasing PTGA content. Such increase should mainly be explained by the plasticizer role of PTGA, thereby increasing the chain mobility of PLLA in the blends. However, PLLA/PTGA blends showed the same crystallization mechanism and crystal structure as neat PLLA, despite the presence of PTGA.
References
Raquez J, Habibi Y, Murariu M, Dubois P. Polylactide (PLA)-based nanocomposites. Prog Polym Sci. 2013;38:1504–42.
Saeidlou S, Huneault M, Li H, Park C. Poly(lactic acid) crystallization. Prog Polym Sci. 2012;37:1657–77.
Yamane H, Sasai K. Effect of the addition of poly(d-lactic acid) on the thermal property of poly(l-lactic acid). Polymer. 2003;44:2569–75.
Tsuji H, Tashiro K, Bouapao L, Narita J. Polyglycolide as a biodegradable nucleating agent for poly(l-lactide). Macromol Mater Eng. 2008;293:947–51.
Xu C, Lv Q, Wu D, Wang Z. Polylactide/cellulose nanocrystal composites: a comparative study on cold and melt crystallization. Cellulose. 2017;24:2163–75.
Qiu Z, Li Z. Effect of orotic acid on the crystallization kinetics and morphology of biodegradable poly(l-lactide) as an efficient nucleating agent. Ind Eng Chem Res. 2011;50:12299–303.
Weng M, Qiu Z. Effect of cyanuric acid on the crystallization kinetics and morphology of biodegradable poly(l-lactide) as an efficient nucleating agent. Thermochim Acta. 2014;577:41–5.
Pan P, Liang Z, Cao A, Inoue Y. layered metal phosphonate reinforced poly(l-lactide) composites with a highly enhanced crystallization rate. ACS Appl Mater Interfaces. 2009;1:402–11.
Pan H, Qiu Z. Biodegradable poly(l-lactide)/polyhedral oligomeric silsesquioxanes nanocomposites: enhanced crystallization, mechanical properties, and hydrolytic degradation. Macromolecules. 2010;43:1499–506.
Zhao Y, Qiu Z, Yang W. Effect of functionalization of multiwalled nanotubes on the crystallization and hydrolytic degradation of biodegradable poly(l-lactide). J Phys Chem B. 2008;112:16461–8.
Qiu Z, Guan W. In situ ring-opening polymerization of poly(l-lactide)-graft-graphene oxide and its effect on the crystallization kinetics and morphology of biodegradable poly(l-lactide) at low loadings. RSC Adv. 2014;4:9463–70.
Martin O, Averous L. Poly(lactic acid): plasticization and properties of biodegradable multiphase systems. Polymer. 2001;42:6209–19.
Kulinski Z, Piorkowska E, Gadzinowska K, Stasiak M. Plasticization of poly(l-lactide) with poly(propylene glycol). Biomacromol. 2006;7:2128–35.
Zhang H, Fang J, Ge H, Han L, Wang X, Hao Y, Hang C, Dong L. Thermal, mechanical, and rheological properties of polylactide/poly(1,2-propylene glycol adipate). Polym Eng Sci. 2013;53:112–8.
He X, Qiu Z. Effect of poly(ethylene adipate) with different molecular weights on the crystallization behavior and mechanical properties of biodegradable poly(l-lactide). Thermochimi Acta. 2018;659:89–95.
Liang H, Hao Y, Liu S, Zhang H, Li Y, Dong L, Zhang H. Thermal, rheological, and mechanical properties of polylactide/poly(diethylene glycol adipate). Polym Bull. 2013;70:3487–500.
Li J, Zhao Y, Jiang Z, Qiu Z. Effect of low molecular weight poly(diethylene glycol adipate) on the crystallization behavior and mechanical properties of biodegradable poly(l-lactide) in their partially miscible blends. Polym Degrad Stabil. 2019;159:217–23.
Yang Y, Qiu Z. Crystallization kinetics and morphology of biodegradable poly(butylene succinate-co-ethylene succinate) copolyesters: effects of comonomer composition and crystallization temperature. CrystEngComm. 2011;13:24082417.
Avrami M. Kinetics of phase change. II. Transformation-time relations for random distribution of nuclei. J Chem Phys. 1940;8:212–24.
Avrami M. Granulation, Phase change, and microstructure kinetics of phase change. III. J Chem Phys. 1941;9:177–84.
Zhang J, Duan Y, Sato H, Tsuji H, Noda I, Yan S, Ozaki Y. Crystal modifications and thermal behavior of poly(l-lactic acid) revealed by infrared spectroscopy. Macromolecules. 2005;38:8012–21.
Acknowledgements
Part of this work was financially supported by the National Natural Science Foundation, China (51573016 and 52173019).
Author information
Authors and Affiliations
Contributions
HZ: investigation, original draft preparation, writing; ZJ: conceptualization, supervision; ZQ: conceptualization, supervision, writing- reviewing and editing.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, H., Jiang, Z. & Qiu, Z. Fully biodegradable poly(l-lactide)/poly(triethylene glycol adipate) blends with different phase behaviors and significantly increased crystallization rate. J Therm Anal Calorim 148, 13385–13391 (2023). https://doi.org/10.1007/s10973-023-12641-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12641-z