Abstract
Microencapsulated ammonium polyphosphate (GMFAPP) is prepared by in situ polymerization method with a shell of poly(ethylene glycol) modified melamine-formaldehyde resin. Due to the presence of shell, GMFAPP shows less size, higher water resistance and flame retardancy in polypropylene (PP) compared with ammonium polyphosphate (APP). The flame retardant action of GMFAPP and APP in PP are studied using LOI, UL-94 and cone calorimeter, and their thermal stability is evaluated by thermogravimetric apparatus. The limiting oxygen index (LOI) value of the PP/GMFAPP at the same loading is higher than the value of PP/APP. UL-94 ratings of PP/GMFAPP can reach V-0 at 30 wt% loading. The water resistant properties of the PP composites are studied, and the results of the composites containing with APP and GMFAPP are compared. The cone results put forward that GMFAPP is an effective flame retardant in PP compared with APP. Moreover, the thermal oxidative behavior of GMFAPP is evaluated by dynamic FTIR to study its flame retardant mechanism in PP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polypropylene (PP) is one of the five kinds of universal polymers and is widely used in a wide variety of applications, such as electrical engineering, housing materials and transport, etc [1–3]. However, flame retardation has become an important requirement in many fields due to easy flammability of neat PP and its polymer alloy.
Among the flame retardants for PP, intumescent flame retardants (IFR) have aroused a great attention in recent years because they are not only more environmentally friendly than the traditional halogen-containing flame retardant but also show higher flame retardant efficiency compared with silicon-containing and metal hydroxides flame retardants [3–6]. In general, a typical IFR system is composed of three components: an acid agent (e.g., ammonium polyphosphate), a carbonization agent (e.g., pentaerythritol) and a blowing agent (e.g., melamine).
Unfortunately, due to their special chemical structure, most IFR systems have some problems such as moisture sensitivity and poor compatibility in polymer matrix. For example, the char agents commonly used in IFR systems are polyols such as pentaerythritol, mannitol or sorbitol, and exudation and higher water solubility are problems associated with these additives [7]. Moreover, the acid agent, APP particle, is also easily attacked by moisture and agglomerate.
In our previous work, APP was microencapsulated with double shell composed of urea-formaldehyde (UF) and melamine-formaldehyde (MF) resin by in situ polymerization method. The double shell outside microencapsulated APP (MUFAPP) decreases its water absorption, and increases its water resistance in PP compared with APP. The limiting oxygen index (LOI) values of the PP/MUFAPP composites are higher than that of PP/APP composites at the same loading. However, due to the weak char capacity of UF, when MUFAPP loading increases to 40%, the UL-94 result of PP/MUFAPP can pass V-0. Moreover, the size of MUFAPP becomes bigger due to the thick double shell [8].
To expand our previous work, we have developed another approach to microencapsulate ammonium polyphosphate with a poly(ethylene glycol) (PEG) modified MF resin shell recently. PEG is an inexpensive and low toxic polymer with many C–O–C and O–H groups. Moreover, PEG is a polyol which may show better char capacity compared with UF. We aim to obtain microcapsule which contains three components of typical IFR system in one: acid agent (APP), carbonization agent (PEG) and blowing agent (melamine). The advantage is to synthesize a flame retardant which shows better compatibility, water resistance, flame retardancy and less size compared with APP and MUFAPP in PP.
In this paper, microencapsulated ammonium polyphosphate (GMFAPP) with a PEG-melamine-formaldehyde (GMF) resin shell was prepared by in situ polymerization and characterized by Fourier transform infrared (FTIR), X-ray photoelectron spectroscopy (XPS), water solubility, laser diffraction particle analyzer and thermogravimetry (TG). The use of GMFAPP as a flame retardant in PP is evaluated by Limiting oxygen index (LOI), UL-94, TG, cone calorimeter and scanning electron microscopy (SEM), and the results from GMFAPP and APP are compared. The water resistant properties of the PP composites containing GMFAPP (or APP) are studied by decrease of LOI and water leaching rate. Moreover, the thermal oxidative behavior of GMF is evaluated by dynamic FTIR.
Experimental
Materials
PP (F401, Mw = 4.2 × 105 g/mol) with a melt flow index (MFI) of 2.3 g/10 min−1 (230 °C/2.16 kg) was provided by Yangzi Petroleum Chemical Company. APP with average degree of polymerization n > 1,000 was provided by Hangzhou JLS Flame Retardants Chemical Corporation. Poly(ethylene glycol) (PEG), a neutral polymer of molecular weight of 1,000 was obtained from Guangdong Guanghua Chemical Factory Co., Ltd, China. Melamine and formaldehyde were purchased from Sinopharm Chemical Reagent Co., Ltd, China.
Preparation of GMFAPP
Synthesis of prepolymer: PEG (6, 9, 12, 15 or 18 g), 4 g melamine and 200 ml distilled water were put into a three-neck bottle with a stir. The mixture was adjusted to pH 4–5 with acetic acid, heated to about 90 °C and kept at that temperature for 1.5 h. Then the pH was adjusted by 10% Na2CO3 solution to 8–9, 4 g melamine and 10 ml 37% formaldehyde solution were added into the system. The temperate was kept at 90 °C for 1 h. The prepolymer solution was prepared and ready for use of the microencapsulation.
Preparation of GMFAPP: 40 g APP was first dispersed in 100 ml ethanol. The prepolymer solution obtained from above step was added into the mixture, and the pH of the mixture was adjusted to 4–5 with sulfuric acid. The resulting mixture was heated at 80 °C for 2 h. After that, the mixture was cooled to room temperature, filtered, washed with distilled water to the neutrality, and dried at 105 °C, and the GMFAPP powder was finally obtained. The proposed reaction scheme of formation of GMF resin is presented in Scheme 1.
Preparation of flame retarded PP composites
All flame retarded PP composites were prepared in a two-roll apparatus at a temperature about 180 °C for 15 min. After mixing, the samples were hot-pressed at about 180 °C under 10 MPa for 10 min into sheets of suitable thickness for analysis.
Measurements
FTIR
Powders were mixed with KBr powders, and the mixture was pressed into a tablet. The Fourier transform infrared (FTIR) spectra of samples were recorded using a Nicolet MAGNA-IR 750 spectrophotometer.
Real time FTIR spectra were recorded using above spectrophotometer equipped with a ventilated oven having a heating device. The temperature of the oven was raised at a heating rate of about 10 °C/min. Dynamic FTIR spectra were obtained in situ during the thermal degradation of the samples.
XPS
The X-ray photoelectron spectroscopy (XPS) spectra were recorded with a VG ESCALAB MK II spectrometer using Al kα excitation radiation (hν = 1,253.6 eV).
Solubility in water
A 10 g sample was put into 100 ml distilled water at different temperature and stirred at that temperature for 60 min. The suspension was then filtered. 50 ml of the filtrate was taken out and dried to constant weight at 105 °C. Solubility of samples in water can be calculated.
Granulometry
The particle size distribution was determined by a laser diffraction particle analyzer (RISE2006, Jinan Rise science Co. Ltd, China). Before the measurement, the samples were dispersed in ethanol, and sonicated for 5 min.
SEM
The SEM micrographs of the PP composites were obtained with a scanning electron microscope AMRAY1000B. The specimens were cryogenically fractured in liquid nitrogen, and then sputter-coated with a conductive layer.
Content of the GMF resin measurement
Few APP or GMFAPP powder was dissolved in nitric acid at 150 °C, and inductively coupled plasma atomic emission spectrometry (Atomscan Advantage, Thermo Jarrell Ash Corporation, USA) was used to measure the phosphorus content of APP or GMFAPP. The symbols PGMFAPP% and PAPP% represent the percentage of phosphorus in GMFAPP and APP, respectively.
Assuming the content of phosphorus remains constant in the process of the microencapsulation of APP, there exist the following equation:
Where MAPP is the content of APP used, and MGMFAPP is the content of GMFAPP obtained. Therefore the percentage of the GMF resin (Wresin wt%) in GMFAPP can be expressed as follows:
If PGMFAPP% and PAPP% are measured, Wresin wt% can be calculated.
Limiting oxygen index
LOI was measured according to ASTM D2863-09. The apparatus used was an HC-2 oxygen index meter (Jiangning Analysis Instrument Company, China). The specimens used for the test were of dimensions100 × 6.5 × 3 mm.
UL- 94 testing
The vertical test was carried out on a CFZ-2-type instrument (Jiangning Analysis Instrument Company, China) according to the UL-94 test standard. The specimens used were of dimensions 130 × 13 × 3 mm.
Determination of water resistance of FR PP composites
The specimens (marked Wa) used for measurement were put in distilled water at 50 °C and was kept at this temperature for 24 h. The treated specimens were subsequently taken out, and dried to constant at 105 °C (marked Wc). The water leaching rate of the specimens can be expressed as (Wa - Wc)/Wa×100%.
Thermogravimetry (TG)
Each sample was examined under air flow on a DTG-60H apparatus (Shimadzu Company) at a heating rate of 10 °C/min.
Cone calorimeter
The combustion tests were performed on the cone calorimeter (Stanton Redcroft, UK) tests according to ISO 5660 standard procedures, with 100 × 100 × 3 specimens. The edges and one face of each specimen were wrapped in an aluminium foil and the other reverse face is exposed horizontally to 35 kW/m2 external heat flux.
Results and discussion
A novel intumescent flame retardant (GMFAPP) composed of APP (core) and GMF resin (shell) is prepared by in situ polymerization. Compared with the results of PP/APP, the effects of GMFAPP on the flame retardant, thermal and water resistant properties of PP composites are studied. Based on these results, the flame retardant mechanism of GMFAPP in PP is discussed.
Characterization of GMFAPP by FTIR and XPS
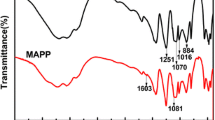
The FTIR spectra of APP and GMFAPP are shown in Fig. 1. It is clear that for GMFAPP, the main absorption peaks appear at 3,200, 1,560, 1,504, 1,339, 1,256, 1,109, 1,075, 1,020 and 880 cm−1. The typical absorption peaks of APP include 3,200 (N–H), 1,256 (P = O), 1,075 (P–O symmetric stretching vibration), 1,020 (symmetric vibration of PO2 and PO3) and 880 (P–O asymmetric stretching vibration) cm−1 [9]. The absorptions of 1,560, 1,504 and 1,339 cm−1 are aroused by the ring vibration of melamine from the MF resin [10]. The peak at 1,109 cm−1 is attributed to the ether bonds (C–O–C) of PEG [11]. The spectrum of GMFAPP reveals not only well-defined absorption peaks of PEG-melamine-formaldehyde (GMF) resin but also the characteristic bands of APP, indicating that the resin exist in the GMFAPP.
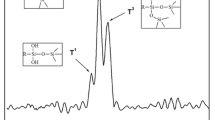
XPS is an effective measurement to study the surface chemical structure of samples without destruction. Figure 2 shows XPS spectra of APP and GMFAPP. The peaks located at 134.7 and 190.9 eV can be assigned to P2P and P2S of APP. After microencapsultion, it is clear that the intensities of corresponding peaks for GMFAPP decrease sharply, meanwhile the intensities of the C1S and N1S peaks increase greatly. The change of the above peaks is aroused by the presence of PGM resin outside GMFAPP particles, indicating that APP was well coated by the shell.
Water solubility of GMFAPP
Figure 3 shows the influence of prepolymer with different content PEG on the water solubility of GMFAPP. From Fig. 3, it can be seen that the solubility of APP at 25 °C and 80 °C is 0.47 and 2.4 g/100 ml H2O, respectively. It is because that APP can be easily attacked by water, especially at high temperatures. After APP particles were microencapsulated by GMF resin, the solubility of GMFAPP decreases to 0.034 g/100 ml H2O at 25 °C, indicating GMF shell outside APP particles is hydrophobic. Moreover, the temperature and PEG content in prepolymer shows few effects on the solubility of GMFAPP, as shown in Fig. 3. Above results also testify that APP is coated well with GMF resin.
Size distribution
The particle size distributions of APP and GMFAPP are shown in Fig. 4. It is clearly seen that the size distribution of GMFAPP is much narrower than that of APP. And the D50 value of GMFAPP is 10.75 μm, much smaller than that of 20.30 μm for APP. This can be explained by that the excellent water resistance of GMF shell which can prevent the core (APP) from the attack of moisture and retard the formation of APP agglomerate. Moreover, due to the single shell outside GMFAPP, it also shows smaller size and narrower size distribution comparing to MUF with double shell [8]. From the difference of size and its distribution, it is expected that GMFAPP would have better dispersion and compatibility in polymers compared with APP or MUFAPP.
Compatibility of APP and GMFAPP in PP
The fractured surface of PP/APP and PP/GMFAPP composites before and after water treatment was observed by SEM, shown in Fig. 5a, b, c and d. The mass percentage of the additives is 30%. Due to the relatively great polarity of APP, their compatibility in PP matrix is not good, lots of grains are exposure on the surface and a clear interfacial line can be observed at the interface. Compared with APP, in Fig. 5c, all GMFAPP embeds in the polymers, which is aroused by the weak polarity and good compatibility of GMF resin with PP.
So when the composites are exposed to water medium, the weak compatibility plus high water solubility of APP may lead grains on the surface dissolve in the water and remove from the matrix. As Fig. 5b shows, after hot water treatment for 24 h, no APP particles can be found on the fracture surface.
In another aspect, after being treated at 50 °C for 24 h, there are still some GMFAPP grains left in PP matrix (Fig. 5d). It can be concluded that microencapsulation have remarkable effect on the dispersion, compatibility and water resistance of APP in PP matrix.
Flame retardation of PP composites
It is suggested that a suitable phosphorus/nitrogen/carbon ratio in the IFR system is very important for its flame retardancy. Therefore, the proportion of GMF (blowing and carbonization agent) and APP (acid agent) in GMFAPP is a key factor which influences the flame retardancy of PP/GMFAPP. In this work, the percentage of shell in GMFAPP is adjusted by changing the content of PEG in the prepolymer. The influence of prepolymer with different content of PEG on the LOI values of PP/GMFAPP composites is shown in Fig. 6. GMFAPP is blend with PP at the mass percentage of 30%. From the figure, it can be seen that with the increase of PEG content, LOI value increase. However, when the content of PEG in prepolymer is above 15 g, the LOI values of PP/GMFAPP changes few. It is hypothesized that a certain melamine can’t react with excess PEG, so when the dosage of PEG is above 15 g, redundant PEG would dissolve in the water without reaction. GMFAPP sample which was microencapsulated by prepolymer containing 15 g PEG was selected for the next step, and from the Eq. 1 in Measurements section, it can be calculated that this GMFAPP sample is coated with 31.6% resin.
APP used alone in PP doesn’t have good flame retardancy (no ratings in the UL-94 test) due to the scarcity of carbonization agent. With the presence of blowing and carbonization agent (GMF resin), the LOI value of PP/GMFAPP (coated with 31.6% resin) is 30.5%, while the value of the PP/APP composite at the same additive level is only 20.0%. Moreover, it should be noticed that the UL-94 results of most PP/GMFAPP composites can reach V-0. Above results illustrate that GMFAPP is an effective flame retardant in PP compared with APP.
Water resistance of FR PP composites
Water leaching rates of PP/APP and PP/GMFAPP versus different PEG content in prepolymer are shown in Fig. 7. The mass percentage of the additives is 30%. It can be seen that through microencapsulation, leaching rate of PP/GMFAPP reduce much, from 9.81% (PP/APP) to 0.23% as the percentage of GMFAPP is 30%. The reason is the good compatibility and excellent water resistance of GMFAPP compared with APP in PP. Moreover, with the increase of PEG content in prepolymer, leaching rates of PP/GMFAPP change little.
The changes of flame retardancy of PP composites containing APP or GMFAPP after the hot water treatment (50 °C, 24 h) are shown in Fig. 6. For the PP/APP composite at 30 wt % loading, its LOI value is 20.0% before the treatment, and the values decrease by 2.5% after the hot water treatment. The LOI value of PP/GMFAPP composite is 30.0% after water treatment at the some additive level. Moreover, though the little decrease of the LOI values for PP/GMFAPP composite is observed, a good maintaining of the UL-94 ratings can be found (most are still V-0 rating). When exposure in water medium, the comparatively better dispersion and excellent water resistance of GMFAPP in PP would prevent the particles from being exuded, and a certain flame retardancy of composite can still be maintained after hot water treatment.
Thermal analysis
Figure 8 presents the TG and DTG curves of APP and GMFAPP. In Fig. 8, it is clear that APP has two main decomposition processes. Its initial temperature is about 270 °C. The gas products in the first step are mainly ammonia and water, and crosslinked polyphosphoric acids (PPA) are formed simultaneously [12]. The main decomposition process of APP occurs above 500 °C. The temperatures of maximum mass loss rate (Tmax) for the two steps are 326 °C and 625 °C, respectively, as shown in Fig. 8b. The residual weight of APP is 2.4% at 700 °C.
GMFAPP begins to degrade at about 245 °C, which is a little lower than that of APP, due to the weak thermal stability of GMF resin. With the increase of temperature, APP release PPA which may react with PEG of GMF. As a result, GMFAPP decomposes faster than APP, as Fig. 8b shows. However, beyond the temperature of 612 °C, GMFAPP are more stable than APP, indicating a char with better thermal stability is formed due to the esterification between APP and GMF. The Tmax values of the main decomposition steps of GMFAPP are 384 and 584 °C, respectively. Moreover, GMFAPP after decomposition at 700 °C left about 27.7% residue, which is much higher than that of APP.
The TG and DTG curves of PP, PP/APP and PP/GMFAPP are shown in Fig. 9. The mass percentage of the additives is 30%. Pure PP begins to decompose at about 240 °C and almost decomposes completely at 360 °C. The Tmax of the decomposition is 299 °C, as shown in Fig. 9a.
The thermal decomposition of the PP/APP composite includes three steps. Its initial decomposition temperature is a bit higher than that of PP. The composite PP/APP decomposes initially at about 250 °C, which is caused by the decomposition of APP. The second step of mass loss is the main decomposition process of PP in the composite, and its Tmax in this step is 366 °C. The third step occurs at above 500 °C due to the further decomposition of the char.
It can be seen in Fig. 9a that the initial decomposition temperature of PP/GMFAPP is similar with that of PP/APP. However, PP/GMFAPP decomposes much fast in comparison with PP/APP at low temperatures, which is aroused by the thermal degradation of GMFAPP. Due to the reaction between the core (APP) and shell (GMF) in GMFAPP, a char with better thermal stability is formed. Therefore, beyond the temperature of 363 °C, PP/GMFAPP is more thermally stable than PP/APP. The Tmax values of the main decomposition steps of PP/GMFAPP are 276, 347 and 613 °C, respectively. The first step is aroused by the degradation of GMFAPP and PP, the second one is due to the formation of a char, and the third peak can be assigned to the further decomposition of the char. The residue left at 700 °C of PP/GMFAPP is 6.8% which is a little higher than that of PP/APP. The increase of amount of residue of the composite is due to the formation of more thermally stable carbonaceous char which is important for the flame retardancy of PP composites in a fire. Above results are according to the data of flame retardation of PP composites.
Cone study
Cone calorimetry is an effective approach to compare the combustion behavior of flame retarded polymers.
The heat release rate (HRR) curves of PP, PP/APP (30 wt%) and PP/GMFAPP (30 wt%) plotted as a function of time are shown in Fig. 10. The HRR curve of PP shows a very intense peak of 1,177 kW/m2 at 136 s, which means that PP is highly flammable. The addition of APP in PP decreases the time-to-ignition (from 44 to 24 s), but also significantly decreases the peak HRR (from 1,177 to 1,064 kW/m2). This indicates that although the sample ignites early, its overall flammability is reduced. The early ignition of PP/APP is aroused by that under cone heater irradiance, the additive decomposes earlier compared with pure PP, and some small flammable gas products are produced. It is reported that many polymer composites containing APP have the similar phenomena [13, 14].
The addition of GMFAPP alone in sample PP/GMFAPP shows remarkable effect on decrease in HRR of PP. It is interesting that the HRR curve of PP/GMFAPP shows a low peak followed by a broad peak, while PP and PP/APP only have one peak. The first peak occurs at 45 s and can be assigned to the ignition and formation of an expanded protective shield. As a result, the presence of GMFAPP in PP also decreases the ignition time (from 44 to 35 s). However, the intumescent char, which has excellent thermal stability and insulation of heat, can prevent the substrate materials from further breakage in a fire. It is the reason that the second peak HRR value of PP/GMFAPP (243 kW/m2) is very lower than that of PP and PP/APP. The second peak is explained by the destruction of the intumescent structure and the formation of a carbonaceous residue. It can be drawn that addition of GMFAPP in PP leads to a delay in the time to ignition and strongly prolongs the process of combustion compared with PP/APP composite.
The appearance of PP/APP and PP/GMFAPP residues at the end of cone calorimeter tests are shown in Fig. 11. It is clear that there is almost no residue left at the end of the cone calorimeter test for PP/APP. On the other hand, the surface of PP/GMFAPP residue is covered with a thick black expanded char network. The conclusion can be drawn that an effective intumescent char layer with good thermal stability can insulated the transfer of heat and substance and prevent the underlying materials from further combustion in a fire.
Thermal oxidative degradation of GMFAPP
In order to study the flame retardant mechanism of GMFAPP, the details of the thermal oxidative behavior of material is revealed by the dynamic FTIR technique, where a sample is heated in an oven to a desired temperature and subjected to FTIR analysis synchronously. Figure 12 shows the dynamic FTIR spectra of GMFAPP.
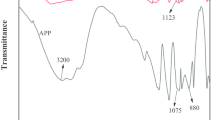
In Fig. 12, the main characteristic absorption bands for GMFAPP show few changes in the temperature range from room temperature to 250 °C. At 300 °C, the peak at 1,434 cm−1 (–NH4) disappears, which means that the degradation of APP and the release of NH3 and PPA [15]. It also can be testified by the movement of the peak at 1,256 cm−1 (P = O) [12]. Moreover, above 300 °C, the peak corresponding to ring vibration (1,560, 1,504 and 1,339 cm−1) of melamine from the GMF resin does not appear, indicating the thermal decomposition of GMF shell [10]. With the increase of temperature, the peaks at around 3,200 cm−1 (–OH and N–H) decrease sharply, which is aroused by the esterification between PPA and PEG in GMF [12, 15]. The occurrence of new peak at 1,150 cm−1 (P–O–C) also testifies this reaction [15]. At higher temperature (above 550 °C), complex compounds containing stable structure (P–O–P and P = O; 1,075, 1,020 and 880 cm−1)) are formed [10].
Conclusion
In this work, APP was microencapsulated with PEG-melamine-formaldehyde resin by in situ polymerization method. Microencapsulated APP (GMFAPP) decreases its water absorption and size distribution, and increases its compatibility and flame retardancy in PP. The LOI value of PP/GMFAPP is higher than that of PP/APP. Moreover, with the presence of shell, GMFAPP used alone in PP can reach V-0 at the additive level 30%. After water treated at 50 °C for 24 h, PP/GMFAPP could still maintain good flame retardant properties (V-0). The TG and cone calorimeter results put forward that GMFAPP is a better flame retardant in PP compared with APP owing to the shell which can be used as blowing and carbonization agent.
From above results, the flame retardant mechanism of GMFAPP in PP can be described as follow: The core (APP) of GMFAPP can release polyphosphoric acid and react with PEG of GMF (shell) to form a char with good thermal stability. On another hand, APP and GMF release a lot of gas, such as NH3 and water vapor, which is helpful in the formation of an intumescent char. The presence of the char with good thermal stability can retard the transfer of heat and substance and prevent underlying materials from further destruction in a fire.
References
Pandey JK, Reddy KR, Kumar AP, Singh RP (2005) Polym Degrad Stab 88:234–250
Chen XL, Yu J, He M, Guo SY, Luo Z, Lu SJ (2009) J Polym Res 16:357–362
Zhang S, Horrocks AR (2003) Prog Polym Sci 28:1517–1538
Wang JC, Yang K, Zheng XY (2009) J Polym Res 16:427–436
Lv P, Wang ZZ, Hu Y, Yu MG (2009) J Polym Res 16:81–89
Chen XL, Jiao CM (2009) J Polym Res 16:537–543
Bourbigot S, Le Bras M, Duquesne S, Rochery M (2004) Macromol Mater Eng 289:499–511
Wu K, Song L, Wang ZZ, Hu Y (2009) J Polym Res 16:283–294
Bugajny M, Bourbigot S, Le Bras M (1999) Polym Int 48:264–270
Wu Q, Lv JP, Qu BJ (2003) Polym Int 52:1326–1331
Luo WJ, Li SM, Bei JZ, Wang SG (2002) J Appl Polym Sci 84:1729–1736
Camino G, Grassie N, McNeill IC (1978) J Polym Sci Pol Chem 16:95–106
Peng HQ, Zhou Q, Wang DY, Chen L, Wang YZ (2008) J Ind Eng Chem 14:589–595
Li B, Jia H, Guan LM, Bing BC, Dai JF (2009) J Appl Polym Sci 114:3626–3635
Colthup NB, Daly LH, Wiberley SE (1990) Introduction to infrared and Raman spectroscopy, 2nd edn. Academic Press, Boston
Acknowledgements
The financial supports from the Teamwork Projects Funded by Guangdong Natural Science Foundation (No. E06200692) and Scientific Research Foundation for Doctor of Guangzhou Institute of Chemistry, Chinese Academy of Sciences (No. QD3) are acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, K., Shen, MM. & Hu, Y. Synthesis of a novel intumescent flame retardant and its flame retardancy in polypropylene. J Polym Res 18, 425–433 (2011). https://doi.org/10.1007/s10965-010-9433-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-010-9433-1