Abstract
Microencapsulated ammonium polyphosphate (MUFAPP) with a double shell is prepared by in situ polymerization, and is characterized by FTIR, XPS and SEM, etc. The microencapsulation of APP can increase its flame retardance and water resistance in PP. The flame retardant action of MUFAPP and APP in PP are studied using LOI and UL 94 test, and their thermal stability is evaluated by TG. The LOI value of the PP/MUFAPP composite at the same loading is higher than that of PP/APP composite. The LOI values of the PP/MUFAPP/ dipentaerythritol are higher than that of the PP/MUFAPP, and UL 94 ratings of most ternary composites are raised to V-0 at 30 wt.% loading. The results of the cone calorimeter also indicate that MUFAPP is an effective flame retardant in PP. The thermal degradation behaviors of APP and MUFAPP are studied using TG and dynamic FTIR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polypropylene (PP) is an important commodity plastic which is widely used in many fields such as wire, cables, automobiles, etc. Many researchers have been interested in the flame retardancy of PP due to its easy flammability [1–7]. Among the flame retardants for PP, intumescent flame retardant (IFR) has aroused a great attention in recent years because they are more environmentally friendly than the traditional halogen-containing flame retardant. Ammonium polyphosphate/melamine/pentaerythritol (APP/MEL/PER) is a typical IFR system and the conventional IFR system is composed of three components: an acid source (e.g. ammonium polyphosphate, etc.), a carbonization agent (e.g. pentaerythritol, dipentaerythritol, sorbitol, etc.) and a blowing agent (e.g. melamine, etc.). Bourbigot and his co-workers have done extensive studies on the APP intumescent flame retardants system in polyolefins [2–5], and reviewed the recent developments of the IFR systems in great detail [1].
Unfortunately, the poor water resistance and compatibility with PP matrix of IFR system restrict its application. Sun et al. found that surface modification of APP particles with surfactants can improve its water resistance [8]. Microencapsulation with water-insoluble polymers is another good choice. Saihi et al. [9, 10] have utilized the technique of coacervation and interfacial polymerization to coat ammonium phosphate (DAHP) with a polyurethane shell in the water solution. In our recent work, we coated APP with melamine–formaldehyde (MF) resin by in situ polymerization method in the ethanol/water medium [11]. Microencapsulated APP (MCAPP) with MF resin shell decreases its water absorption, and increases its water resistance in PP matrix. Though LOI values of the PP/MCAPP composites increases, it has been found that MCAPP used alone in PP does not pass any rating in UL 94 test because the scarcity of carbonization agents. Though the addition of PER can increase the flame retardance of PP composites, it may reduce the water resistance of PP composites due to the high water solubility of PER.
It is reported that urea–formaldehyde (UF) is helpful in the formation of residues on heating which can protect underlying materials from further burning [12]. But the water resistance of UF resin is weaker compared with MF resin. As a result, we microencapsulated APP with UF resin as primary layer, and then coated the particles with MF resin compactly. The double shell outside APP particles can be used as a protective layer and carbonization or blowing agent synchronously. Therefore, the advantage of this work was to obtain a different intumescent flame retardant which has better flame retardant and higher water resistant properties compared with conventional APP IFR systems in PP.
In this work, microencapsulated ammonium polyphosphate (MUFAPP) with a double shell (MF resin and UF resin) was prepared by in situ polymerization and characterized by water solubility, Fourier transform infrared (FTIR), thermogravimetry (TG) and X-ray photoelectron spectroscopy (XPS). The use of MUFAPP as a flame retardant in PP is evaluated by limiting oxygen index (LOI), UL-94, TG, cone calorimeter and scanning electron microscopy (SEM), and the results from MUFAPP and APP are compared. The water resistant properties of the PP composites containing MUFAPP (or APP) and dipentaerythritol is studied by the water leaching rate and decrease of LOI value. Moreover, the thermal degradation of MUFAPP is evaluated using dynamic FTIR.
Experimental
Materials
PP (F401) with a melt flow index (MFI) of 2.3 g/10 min−1 (230 °C/2.16 kg) was provided by Yangzi Petroleum Chemical Company. APP with average degree of polymerization n > 1,000 was kindly supplied by Hangzhou JLS Flame Retardants Chemical Corporation. Urea, melamine and formaldehyde were chemical reagents purchased from Shanghai Chemical Reagent Corporation. Dipentaerythritol (DPER) was provided by Puyang Yongan Chemical Corporation.
Preparation of microencapsulated APP
Synthesis of UF resin prepolymer
10 g urea, 37% formaldehyde solution (with a mole ratio of 1:2.2) and 50 ml distilled water were put into a three-neck bottle with a stir. The mixture was adjusted to pH 8–9 with 10% Na2CO3 solution, heated to about 80 °C and kept at that temperature for 1 h. The UF prepolymer solution was prepared to 100 ml with distilled water and ready for the microencapsulation. Figure 1 shows the reaction scheme of the formation of UF resin prepolymer [13].
Synthesis of MF resin prepolymer
Ten grams melamine, 37% formaldehyde solution (with a mole ratio of 1:3.0) and 50 ml distilled water were put into a three-neck bottle with a stir. The mixture was adjusted to pH 8–9 with 10% Na2CO3 solution, heated to about 80 °C and kept at that temperature for 1 h. The MF prepolymer solution was prepared to 100 ml with distilled water and ready for the microencapsulation. Figure 2 shows the reaction scheme of the formation of MF resin prepolymer [14].
Preparation of microencapsulated APP
Forty grams APP was first dispersed in 100 ml ethanol. Then suitable amount of UF prepolymer solution was added into the mixture, and the pH of the mixture was adjusted to 4–5 with sulfuric acid. The resulting mixture was heated at 80 °C for 2 h. Then 32 ml MF prepolymer solution was added into the system and the PH was adjusted to 4–5. The temperature of system was kept at 80 °C for 2 h. After that, the mixture was filtered, washed with distilled water, and dried at 105 °C, and the MUFAPP powder was finally obtained. Figures 1 and 2 show the condensation reaction scheme of UF and MF resins [13, 15].
Preparation of flame retarded PP composites
All flame retarded PP composites were prepared in a Brabender-like apparatus at a temperature about 180 °C for 15 min. After mixing, the samples were hot-pressed at about 180 °C under 10 MPa for 10 min into sheets of suitable thickness for analysis. The formulations are given in Table 1.
Measurements
Fourier transform infrared spectra
Powders were mixed with KBr powders, and the mixture was pressed into a tablet. The Fourier transform infrared (FTIR) spectra of samples were recorded using a Nicolet MAGNA-IR 750 spectrophotometer.
Real time FTIR spectra were recorded using above spectrophotometer equipped with a ventilated oven having a heating device. The temperature of the oven was raised at a heating rate of about 10 °C/min. Dynamic FTIR spectra were obtained in situ during the thermal degradation of the samples.
X-ray photoelectron spectroscopy spectra
The X-ray photoelectron spectroscopy (XPS) spectra were recorded with a VG ESCALAB MK II spectrometer using Al kα excitation radiation (hν = 1,253.6 eV).
Granulometry
The particle size distribution was determined by a laser diffraction particle analyzer (RISE2006, Jinan Rise science Co. Ltd, China). Before the measurement, the samples were dispersed in ethanol, and sonicated for 10 min.
Solubility in water
The sample (about 10 g) was put into 100 ml distilled water at different temperature and stirred at that temperature for 60 min. The suspension was then filtered. Fifty milliliters of the filtrate was taken out and dried to constant weight at 105 °C. Solubility of samples in water can be calculated.
Scanning electron microscopy
The SEM micrographs of the particles and PP composites were obtained with a scanning electron microscope AMRAY1000B. The particles were sprinkled onto a double-sided tape, sputter coated with gold layer. The composites were cryogenically fractured in liquid nitrogen, and then sputter coated with a conductive layer.
Content of the MUF resin measurement
Few APP or MUFAPP powder was dissolved in nitric acid at 150 °C, and inductively coupled plasma atomic emission spectrometry (Atomscan Advantage, Thermo Jarrell Ash Corporation, USA) was used to measure the phosphorus content of APP or MUFAPP. The symbols P MUFAPP% and P APP% represent the percentage of phosphorus in MUFAPP and APP, respectively.
Assuming the content of phosphorus remains constant in the process of the microencapsulation of APP, there exists following equation:
where M APP is the content of APP used, and M MUFAPP is the content of MUFAPP obtained. Therefore the percentage of the MUF resin (W resin wt.%) in MUFAPP can be expressed as follows:
if P MUFAPP% and P APP% are measured, W resin wt.% can be calculated.
Limiting oxygen index
LOI was measured according to ASTM D2863. The apparatus used was an HC-2 oxygen index meter (Jiangning Analysis Instrument Company, China). The specimens used for the test were of dimensions 100 × 6.5 × 3 mm.
UL- 94 testing
The vertical test was carried out on a CFZ-2-type instrument (Jiangning Analysis Instrument Company) according to the UL 94 test standard. The specimens used were of dimensions 130 × 13 × 3 mm.
Water leaching rate of FR PP composites
The specimens (marked Wa) used for measurement were put in distilled water at 50 °C and was kept at this temperature for 24 h. The treated specimens were subsequently taken out, and dried to constant weight at 80 °C (marked Wc). The water leaching rate of the specimens can be expressed as (Wa − Wc)/Wa × 100%.
Thermogravimetry (TG)
Each sample was examined under air flow on a DTG-60H apparatus (Shimadzu Company) at a heating rate of 10 °C/min.
Cone calorimeter
The combustion tests were performed on the cone calorimeter (Stanton Redcroft, UK) tests according to ISO 5660 standard procedures, with 100 × 100 × 3 specimens. Each specimen was wrapped in an aluminium foil and exposed horizontally to 35 kW/m2 external heat flux.
Results and discussion
FTIR and XPS
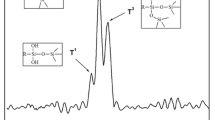
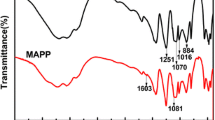
The FTIR spectra of APP and MUFAPP are shown in Fig. 3. The spectrum of MUFAPP show absorption bands at 3,200, 1,649, 1,560, 1,256, 1,075, 1,020 and 880 cm−1. The typical absorption peaks of APP include 3,200 (N–H), 1256 (P = O), 1,075 (P–O symmetric stretching vibration), 880 (P–O asymmetric stretching vibration) and 1,020 (symmetric vibration of PO2 and PO3) cm−1 [16]. The C = O stretching vibration of –N–(CO)–N– of UF resin absorbs at 1,649 cm−1 [18]. The absorption of 1,560 cm−1 is due to the ring vibration of melamine groups from the MF resin [17]. It is clear that not only the absorption peaks of MF and UF resin but also the characteristic bands of APP appear on the FTIR spectra of MUFAPP. Above results mean that the MF and UF resin exist in the MUFAPP.
The core−shell structure of MUFAPP is further revealed by the XPS spectra of APP and MUFAPP in Fig. 4. The peaks located at 134.7 and 190.9 eV are the typical chemical shifts of P2P and P2S in APP. For MUFAPP, the intensities of peaks aforementioned decrease sharply, meanwhile the intensities of the C1S and N1S peaks increase greatly. This might result from the fact that the APP particles are well coated by MF and UF resin.
Size distribution and morphology
The particle size distributions of APP and MUFAPP are shown in Fig. 5. Due to the existence of shell, the D50 value of MUFAPP is 24.623 μm, bigger than APP’s 20.296 μm. It also can be found that the size distribution of MUFAPP is a little wider than that of APP.
Figure 6 shows the surface morphologies of APP and MUFAPP. It can be seen that the surface of APP particle is very smooth, as Fig. 6a shows. After microencapsulation, MUFAPP presents a comparably rough surface. And it is interesting to find that the shell outside particle is similar to a multilayer structure.
Above results also suggest the coating of APP with the UF and MF resin.
Water solubility of MUFAPP
Figure 7 shows the influence of UF prepolymer volume used in the microencapsulation on the water solubility of MUFAPP. From Fig. 7, it can be seen that the solubility of APP without microencapsulation at 25 and 80 °C is 0.43 and 2.4 g/100 ml H2O, respectively. After the microencapsulation of APP with MF and UF resin, the solubility of MUFAPP decreases sharply especially at high temperature. It is clearly seen that the water solubility of APP rises quickly with the increase of temperature. However, the difference of solubility of MUFAPP at 25 and 80 °C is small. As the content of the coated UF resin increases further, the solubility of MUFAPP changes little. The presences of the shell protect the APP particles from being attacked by the water and the MF resin outside APP is hydrophobic, so the solubility of APP decreases. Above results also demonstrate that APP was well coated by the resin.
Flame retardation of PP composites
The influence of UF prepolymer volume used in the microencapsulation on the LOI values of PP/MUFAPP composites is shown in Fig. 8. MUFAPP is blend with PP at the mass percentage of 30%.When more UF prepolymer was used, the MUFAPP sample would be coated with more resin. From the figure, it can be seen that with the increase of UF prepolymer content, the LOI values of PP/MUFAPP composites reach a peak value (31%). It is proposed that a suitable phosphorus/nitrogen/carbon ratio in the IFR system is very important for the flame retardant action of IFR in polymers. Moreover, when 90 ml UF prepolymer was used in the preparation of MUFAPP, the UL 94 result of the PP/MUFAPP can reach V-1. So this MUFAPP sample was selected for the next step, and from the equation in the part of “Content of the MUF resin measurement”, it can be calculated that this MUFAPP sample is coated with 27.1% resin.
The LOI values and UL 94 testing results of the FR PP composites are presented in Table 2. It can be seen that the LOI values of PP/MUFAPP composites are higher than that of PP/APP composites. For example, the LOI value of the composite containing 30% MUFAPP (coated with 27.1% resin) is 31%, while the value of the PP/APP composite is only 20%. The explanation for the increase may be due to the fact that when the PP composites containing MUFAPP are heated, the resin in the coating layer of MUFAPP releases water vapor and NH3 gases which would reduce the concentration of air and make the char swell to form protective char. When MUFAPP loading increases to 40%, the UL-94 result of PPMUFAPP40 is raised to V-0. Above results illustrate that APP used alone do not have good flame retardancy (no ratings in the UL-94 test) compared with MUFAPP in PP, the reason for this is due to the scarcity of carbonization and blowing agent.
Because of the high water solubility of PER, dipentaerythritol (DPER) was used to substitute PER as carbonization agent. When DPER is incorporated into the PP/MUFAPP composites, improvement of flame retardation is observed. The values of the PP/MUFAPP/DPER composites (PPMUFAPP1- PPMUFAPP2) containing 7.5% and 15% DPER are 34.5%, and 33.5%, meanwhile the LOI value of the binary composite (30% MUFAPP) is just 31%. Moreover, the UL-94 ratings for most of PP/MUAPP/DPER at the same additive level (30%) are raised to V-0. Above data indicate that DPER have remarkable effect on the flame retardation of the PP/MUAPP binary composites.
Water resistance of FR PP composites
The water leaching rate of FR PP composites are shown in Fig. 9. It is clearly seen that with the increase of mass percentage of APP, leaching rate of PP/APP/DPER composites suffer a fast increase. This result demonstrates that the APP has weaker water resistance compared with DPER in PP. From Fig. 9 it can be found that through microencapsulation, the leaching rate of FR PP composites reduce much, from 9.81% to 0.1% as the percentage of MUFAPP is 30%. Due to the hydrophobicity of MF resin, with the increase of MUFAPP content, the leaching rates of PP/MUF/DPER composites decrease. So when the FR composites are exposed in water medium, the comparatively better water resistance of MUFAPP would prevent IFRs from being exuded, and a certain flame retardancy of composite can still be maintained.
The changes of flame retardation of the PP composites after the hot water treatment (50 °C, 24 h) are listed in Table 2. For the PP/APP binary composite at 30% additive level, its LOI value is 20% before the treatment, and the values decrease by 2.5% after the hot water treatment. The LOI value of PP/MUFAPP composite at a loading 30% is 31%, whereas the value is still as high as 30% after the treatment. In the case of the PP/APP/DPER composites, the treatment results in not only the decrease of the LOI value, but also their UL-94 ratings. The LOI values of samples PPADPER1 and PPADPER2 are 27.5% and 28%, respectively, and the values decrease to 22.5% and 23%, respectively after the water treatment. Their UL-94 ratings change from V-1 (PPADPER2) to no rating after the treatment. Compared with PP/APP/DPER composites, the water treatment has less impact on the LOI values and UL-94 ratings of the PP/MUFAPP/DPER composites. In spite of the decrease in the LOI values of the PP/MUFAPP/DPER composites after treated, a good maintaining of the UL-94 ratings is observed (still V-0 rating). Therefore, conclusion can be drawn that the water resistance of MUFAPP is much better than APP in PP composites.
The fractured surface of PP/APP and PP/MUFAPP composites before and after water treatment was observed by SEM, shown in Fig. 10a–d. Before water treated, APP grains are distributed unevenly in PP matrix and their compatibility with PP matrix is not good, lots of grains are exposure on the surface, a clear interfacial line can be observed at the interface due to the relatively great polarity of APP. So when the composites are exposed to water medium, the water molecules will absorb on the surface of the material, and some APP grains on the surface will dissolve in the water, leaving some defects on the surface. For PP/MUFAPP composites, after treated at 50 °C for 24 h, there are still some MUFAPP grains left in PP matrix. From these results, it can be concluded that the influence of microencapsulation on the water resistance of APP in FR PP composites is remarkable.
Thermal analysis
The TG and DTG curves of APP and MUFAPP are shown in Fig. 11. APP has two main decomposition processes. It begins to decompose at about 270 °C. The evolution products in the first process are mainly ammonia and water, and cross-linked poly-phosphoric acids (PPA) are formed simultaneously [19]. The second process occurs above 500 °C, which is the main decomposition process of APP. The temperatures of maximum mass loss rate (T max) for the two steps are 326 and 625 °C, respectively, as shown in Fig. 11b. The residue of APP at 800 °C is only about 0.6%.
For MUFAPP, its initial decomposition temperature is 220 °C which is lower than that of APP. In the first process, MUFAPP decomposes faster than APP owing to the less thermal stability of UF or MF resin in MUFAPP [20, 21]. Beyond the temperature of 630 °C, MUFAPP is more stable than APP. From Fig. 11b it can be seen that the degradation of MUFAPP is composed of four steps. The T max for the four steps are 278, 326, 388 and 582 °C, respectively. Moreover, MUFAPP after decomposition at 800 °C left about 1.3% residue, which is higher than that of APP.
The TG and DTG curves of PP and the PP composites are shown in Fig. 12. It is clearly seen that the pure PP begins to decompose at about 240 °C and almost decomposes completely at 360 °C. The T max for the decomposition is 299 °C, as shown in Fig. 12b.
The thermal decomposition of the PP/APP composite (sample PPAPP) includes three steps. Its initial decomposition temperature is a bit higher than that of PP. The composite PPAPP decomposes initially at about 250 °C, which is caused by the decomposition of APP. The second step of mass loss is the main decomposition process of the composite, and its T max for this step is 366 °C. The third step occurs at above 500 °C due to the further decomposition of the char. In comparison with PPAPP, the initial decomposition of sample PPMUFAPP is advanced. However, the TG curve of sample PPMUFAPP moves to a higher temperature side above 358 °C. The T max values for the first three decomposition steps of sample PPMUFAPP are 266, 346, and 401 °C, respectively. Above 570 °C is the fourth step which is the decomposition of the char. It is noticeable that after decomposition at 800 °C PPMUFAPP left about 15% residue, which is much higher than that of PPAPP.
Remarkable different thermal decomposition between binary and ternary composites can be found, when DPER substitutes for some content of APP or MUFAPP in the PP/APP (MUFAPP) composites. As shown in Fig. 12a, the initial decomposition temperatures of the ternary composites PPADPER1 and PPMUFAPP1 are lower than the ones of the PP/APP (or PP/MUFAPP) binary composites because of the weak thermal stability of DPER. Moreover, due to the esterification between acid source and carbonisation agent, PPADPER1 and PPMUFAPP1 decompose much faster than the binary composites (PP/APP or PP/MUFAPP) at low temperatures. However, at the temperature higher than 715 °C, the ternary composite (PPMUFAPP1) is more thermally stable than the binary composites (PPMUFAPP). The residues left at 800 °C for PPADPER1 and PPMUFAPP1 are about 5% and 16.5%, respectively. It can be seen that MUFAPP is better than APP in improving the thermal stability of the PP binary composites or ternary composites at high temperature. The increase of amount of residue of the ternary composites may be due to the formation of more thermally stable carbonaceous char [22].
Cone calorimeter study
Cone calorimetry is an effective approach to evaluate the combustion behavior of flame retarded polymers. Heat Release Rate (HRR) results are shown in Fig. 13. The presence of intumescent systems in PP decreases the HRR values strongly when compared to the pure PP (the HRR peak value of PP is 1177 kW/m2). In case of the PP/APP composite, its HRR peak is behind of that of pure PP, and its value is a little lower (1064 kW/m2) than that of PP. However, it is noted that the ignition time (IT) of the PP/APP composite (24 s) is decrease in comparison with that of PP (44 s). The reason may be due to the fact that APP decomposes earlier than pure PP after the cone heater irradiated the surface of the composite, and some small volatile molecules are produced from the decomposition of APP. Moreover, the HRR curve of PP/APP/DPER is flat and the HRR peak value only reach 566 kW/m2. It is noteworthy that the HRR curve of the PP/APP/DPER is typical one of intumescent systems. This HRR curve exhibits two peaks. The first peak is assigned to the ignition and to the formation of an expanded protective shield. The second peak is explained by the destruction of the intumescent structure and the formation of a carbonaceous residue [1].
Addition of MUFAPP in PP strongly decreases the HRR value and leads to a delay in the time to ignition compared with APP in PP. Associated data for the PPMUFAPP are: IT = 27 s, peak HRR = 375 kW/m2. The results are consistent with the data of LOI and UL 94. The blend of DPER in PP binary composite containing MUFAPP lead the HRR peak value decrease further (356 kW/m2) and prolong the process of combustion. From Fig. 13, it can be seen that after the MUFAPP loading increase to 40%, the curves of PPUMFAPP40 became more flat and shows a further decrease in HRR peak value compared with that of sample PPAPP and PPMUFAPP. Associated data for the PPMUFAPP40 are: IT = 32 s, peak HRR = 233 kW/m2. From these data, it can be concluded that the microencapsulation can reduce the peak HRR value of PP/APP or PP/APP/DPER system remarkably, and the value of HRR is an important criterion in the evaluation of flame retardation of polymers.
The photographs of the PP composite residues at the end of cone calorimeter tests are shown in Fig. 14. It is clear that there is almost no residue left at the end of the cone calorimeter test for PPAPP composite. On the other hand, the surface of PPADPER1 residue is covered with an expanded char network. The residue left by PPMUFAPP is mainly formed of thin black char, and the char is better than that of PPAPP in protecting the underlying materials. With the increase of MUFAPP loading (from 30% to 40%), the char left becomes more coherent and thicker. After the burning, PPMUFAPP1 can form a blacker and thicker char compared with that of PPAPP, PPADEPR1 and PPMUFAPP. It can be concluded that a good and coherent char can prevent the heat transfer and flame spread, and thus protect the underlying materials from further burning.
Thermal degradation of MUFAPP
To study the flame retardant mechanism of MUFAPP in polymers, we used dynamic FTIR to evaluate the thermal degradation of MUFAPP.
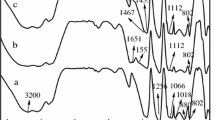
For MUFAPP (Fig. 15), no modification of the chemical structure is observed below 250 °C. With the increase of temperature, above 250 °C, the bands which correspond to –NH4 (1,434 cm−1) [23] disappear; this may be related with the elimination of NH3. Between the temperature of 25–300 °C, as the pyrolysis temperature increases, the 1,256 cm−1 peak (P = O) move to a higher waver number [16]. The reason for the movement may be the scission of P–O–N and the elimination of NH3. Moreover, we should notice the absence of the absorptions of 1,649 and 1,560 cm−1 which are due to C = O stretching vibration and the ring vibration of UF and MF [17, 18] at about 300 °C; it can be explained by the degradation of shell outside APP. These results demonstrate that the evolution products in the first process are mainly ammonia and water, and crosslinked polyphosphoric acids (PPA) are formed simultaneously. It is interesting to find that between the range of 300 and 600 °C, the shape of spectra show few change. It may be related with the formation of stable structures containing P–O–P and P = O (1,075, 1,020, 880 cm−1) complexes [16].
These dynamic thermal degradation data give positive evidences of the flame retardant mechanism: MUFAPP can release the acid and form a stable charred layer in the condensed phase during burning of polymer materials. These char slow down heat and mass transfer between the gas and condensed phases and prevent the underlying polymeric from further combust. These results are in agreement with the data of TG, LOI, UL 94 and cone calorimeter.
Conclusion
In this work, APP was microencapsulated with double shell composed of urea–formaldehyde and melamine–formaldehyde resin by in situ polymerization method. Microencapsulated APP (MUFAPP) decreases its water absorption, and increases its water resistance in PP matrix. The LOI values of the PP/MUFAPP composites increase compared with the ones of the PP/APP composites at the same loading. It has been found that APP used alone in PP does not pass any rating and MUFAPP used alone in PP can reach V-1 in UL 94 test at a loading 30%. When MUFAPP loading increases to 40%, the UL-94 result of PP/MUFAPP (PPMUFAPP40) is raised to V-0. Above results indicate that the double shell lead MUFAPP a higher water resistance and better flame retardance compared with APP in PP.
After dipentaerythritol (DPER) is incorporated into the PP/MUAPP or PP/APP composites, a remarkable improvement of flame retardation is observed, not only the increase in LOI value, but the UL-94 V-0 ratings reached for some of PP/MUFAPP/DPER composites at a loading 30%. It also can be found that after water treated at 50 °C, the composites containing MUFAPP could still maintain good flame retardant properties. The TG and cone calorimeter results also indicate that MUFAPP is an effective flame retardant compared with APP in PP.
The thermal degradation of MUFAPP is evaluated by TG and dynamic FTIR. The results show that MUFAPP can form a stable charred layer in the condensed phase and prevent the underlying polymeric from further combust during burning.
References
Bourbigot S, Le Bras M, Duquesne S, Rochery M (2004) Macromol Mater Eng 289:499 doi:10.1002/mame.200400007
Le Bras M, Bourbigot S, Delporate C, Siat C, Le Tallec Y (1996) Fire Mater 20:191 doi:10.1002/(SICI)1099-1018(199607)20:4<191::AID-FAM577>3.0.CO;2-S
Almeras X, Le Bras M, Hornsby P, Bourbigot S, Marosi G, Keszei S et al (2003) Polym Degrad Stabil 82:325 doi:10.1016/S0141-3910(03)00187-3
Almeras X, Le Bras M, Poutch F, Bourbigot S, Marosi G, Anna P (2003) Macromol Symp 198:435 doi:10.1002/masy.200350837
Le Bras M, Bugajny M, Lefebvre JM, Bourbigot S (2000) Polym Int 49:1115 doi:10.1002/1097-0126(200010)49:10<1115::AID-PI430>3.0.CO;2-9
Metin D, Tihminlioglu F, Balkose D, Ulku S (2004) Compos Part A. Appl Sci Manufactur 35:23 doi:10.1016/j.compositesa.2003.09.021
Wu Q, Qu BJ (2001) Polym Degrad Stabil 74:255 doi:10.1016/S0141-3910(01)00155-0
Sun CY, Zhang QB, Li B (2007) J Polym Res 14:505 doi:10.1007/s10965-007-9135-5
Saihi D, Vroman I, Giraud S, Bourbigot S (2005) React Funct Polym 64:127 doi:10.1016/j.reactfunctpolym.2005.05.004
Saihi D, Vroman I, Giraud S, Bourbigot S (2006) React Funct Polym 66:1118 doi:10.1016/j.reactfunctpolym.2006.02.001
Wu K, Wang ZZ, Liang HJ (2008) Microencapsulation of Ammonium Polyphosphate: Preparation, Characterization and its flame retardance in Polypropylene. Polym Compos doi: 10.1002/pc.20459
Garba B, Eboatu AN, Abdulrahman FW (1996) Fire Mater 20:155 doi:10.1002/(SICI)1099-1018(199605)20:3<155::AID-FAM557>3.0.CO;2-R
Yuan L, Liang GZ, Xie JQ, Li L, Guo J (2006) Polymer (Guildf) 47:5338 doi:10.1016/j.polymer.2006.05.051
Lee YH, Kim CA, Jang WH, Choi HJ, Jhon MS (2001) Polymer (Guildf) 42:8277 doi:10.1016/S0032-3861(01)00342-1
He XD, Ge XW, Wang MZ, Zhang ZC (2005) Polymer (Guildf) 46:7598 doi:10.1016/j.polymer.2005.06.015
Bugajny M, Bourbigot S, Le Bras M (1999) Polym Int 48:264 doi:10.1002/(SICI)1097-0126(199904)48:4<264::AID-PI118>3.0.CO;2-M
Wu Q, Lv JP, Qu BJ (2003) Polym Int 52:1326 doi:10.1002/pi.1115
Kandelbauer A, Despres A, Pizzi A, Taudes I (2007) J Appl Polym Sci 106:2192 doi:10.1002/app.26757
Camino G, Grassie N, McNeill IC (1978) J Polym Sci: Polym Chem Ed 16:95
Kay M, Price AF, Lavery I (1979) J Fire Retardant Chem 6:69
Broadbent JRA, Hirschler MM (1984) Eur Polym J 20:1087 doi:10.1016/0014-3057(84)90134-4
Bourbigot S, Le Bras M (1995) Carbon 33:283 doi:10.1016/0008-6223(94)00131-I
Colthup NB, Daly LH, Wiberley SE (1990) Introduction to infrared and Raman spectroscopy. Academic Press, Boston
Acknowledgement
The financial support from the National Natural Science Foundation of China (no. 20776136), the program for New Century Excellent Talents in University and National 11th Five-year Program (2006BAK01B03, 2006BAK06B06, 2006BAK06B07) is acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wu, K., Song, L., Wang, Z. et al. Preparation and characterization of double shell microencapsulated ammonium polyphosphate and its flame retardance in polypropylene. J Polym Res 16, 283–294 (2009). https://doi.org/10.1007/s10965-008-9228-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-008-9228-9