Abstract
The effects of hydroxy silicone oil as a synergistic agent on the flame retardancy of intumescent flame retardant polypropylene composites (IFR-PP) were studied, and the IFR system mainly consisted of the ammonium polyphosphate (APP), melamine (MEL) and pentaerythritol (PER). The UL 94 rating, thermogravimetric analysis (TGA), cone calorimeter (CONE) and digital photograph were used to evaluate the synergistic effects of hydroxy silicone oil (HSO). It has been found that the PP composite containing only APP, MEL and PER does not show good flame retardancy at 30% additive level. The cone calorimeter results show that the heat release rate, mass loss rate, mass, total heat release, carbon monoxide and carbon dioxide of PP/APP/MEL/PER/HSO composites decrease in comparison with the PP/APP/MEL/PER composite. The digital photographs demonstrated that HSO could promote to form the homogenous and compact intumescent char layer. Thus, a suitable amount of HSO plays a synergistic effect in the flame retardancy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polypropylene (PP) is used in many fields, such as automobiles, furniture, electronic casings, interior decoration, architectural material. However, due to its chemical constitution, the polymer is easily flammable and so flame retardancy becomes an important requirement for PP. Traditionally, halogen containing compounds, alone or in conjunction with antimony trioxide, are the main flame retardants for PP. However, the use of these flame retardants has been limited for the consideration of life safety and environmental problems [1]. Therefore, it is worthwhile to investigate the halogen-free flame retardation of PP. The compounds used as halogen-free flame retardants in PP include metal hydroxides, phosphorous-containing compounds, phosphorous and nitrogen-containing compounds, etc. Furthermore, metal hydroxides are widely used as flame retardant additives in polypropylene, but the high loading seriously destroys the mechanical properties of polymeric materials [2–5].
In recent years, intumescent flame retardant (IFR) [6–20] is well known as a new generation of flame retardants in polypropylene and other polyolefins for some of their merits, such as very low smoke and toxic gases produced during burning, and antidripping property, which conform to the tendency of flame retardants’ development.
However, it has also some drawbacks compared with bromine-containing flame retardants [21], such as low flame retardant efficiency and low thermal stability. In order to enhance the more effective flame retardancy, new intumescent flame retardant systems have been found [22–25], and synergistic agents have been used in IFR systems, such as zeolites [26, 27], montmorillonite [28, 29], organoboron siloxane [30, 31], and some transitional metal oxides and metal compounds [32–34]. Many researches have shown that synergistic agents can effectively increase the strength and stability of char layer by Si–O–P–C and Al–O–P–C bonds [26, 35], and promote catalyzing the reactions among IFR components in IFR-PP systems. Up to now, no hydroxy silicone oil has been found capable of improving the flame retardance of plastics.
In this work, a most common silicon compound, hydroxy silicone oil, is selected to investigate the synergistic effect in PP-IFR composites, it is used in a IFR system consisting of ammonium polyphosphate (APP), melamine (MEL) and pentaerythritol (PER). The vertical burning test (UL-94), thermogravimetry analysis (TGA), cone calorimeter (CONE) and digital photographs are used to evaluate the synergistic effect of HSO in PP-IFR systems.
Experimental section
Materials
Polypropylene (F401) was provided by Yangzi Petroleum Chemical Company. APP, MEL and PER was supplied by Hefei Keyan Institute of Chemical Engineering; the intumescent flame retardant (IFR) was obtained with the mass ratio of APP, MEL and PER is 2:1:1. Hydroxy silicone oil (QLS-203) was supplied by Wuxi Quanli Reagent Chemical Factory. The formulations are given in Table 1.
Preparation of samples
All samples were prepared on a two-roll mill at a temperature range of 170–175 °C for 15 min. After mixing, the samples were hot-pressed under 10 MPa for 5 min at about 175 °C into sheets of suitable thickness and size for analysis.
Measurements
UL 94 testing
The vertical test was carried out on a CFZ-2-type instrument (Jiangning Analysis Instrument Company, China) according to the UL 94 test standard. The specimens used were of dimensions 130 × 13 × 3 mm3.
Cone calorimeter
The cone calorimeter (Stanton Redcroft, UK) tests were performed according to ISO 5660 standard procedures. Each specimen of dimensions 100 × 100 × 3 mm3 was wrapped in aluminium foil and exposed horizontally to an external heat flux of 35 kW/m2.
Thermogravimetry (TG)
Each sample was examined under nitrogen flow on a STA 409C TGA apparatus (Netzsch Company, Germany) with crucible sample holders, at a heating rate of 10 °C/min.
Results and discussion
Cone calorimeter study
Cone calorimeter is one of the most effective bench scale methods for studying the flammability properties of materials. The heat release rate (HRR), particularly the peak HRR, has been found to be the most important parameter for evaluating fire safety [36–38]. The flammability properties of flame retardant PP composites have been studied using cone calorimeter. Although intumescent flame retardant is commonly used for PP for fire safety materials, sometimes the high loading can deteriorate the mechanical properties of materials. It is necessary, therefore, to develop novel synergistic flame-retardant systems with high efficiency and acceptable environmental impact.
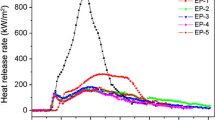
In this study, HSO is added into PP/IFR composites as synergism. The flammability properties were evaluated with cone calorimeter experiments. In the flame retardant PP/IFR/HSO composites, the mass fraction of IFR and HSO was 30 wt.%. The HRR curves are shown in Fig. 1. Figure 1 shows that when 30 wt.% IFR was added to pure PP, the peak HRR was 290 kw/m2. When the 5 wt.% IFR was substituted with HSO (PP5), the curves show that the peak HRR of the sample was 192 kw/m2, which was lowest of the samples. In Fig. 1, a synergistic effect can be seen between IFR and HSO. In this system, the chemical reactions are complex. In IFR systems [39], APP is used as the acid source; during heating, the poly(phosphoric acid) formed in the degradation of APP provides an acid catalyst for organic reactions [8]. During the heating process of APP, ammonia is the main gaseous product, which volatilizes and makes the mixture of the carbonaceous residue and phosphor-carbonaceous materials swell; this leads to the formation of the intumescent char residue. When HSO is added to PP/IFR composites, in addition to the aforementioned chemical reaction, APP reacts with HSO to form a silicon-phosphate structure and a ceramic-like structure in the 310–560 °C temperature range [40]. These silicon-phosphate species may thermally stabilize and lead to good fire performance in this temperature range. On the other hand, there is a catalytic role played by the SiO2 solid acid from the HSO. The decomposition of the APP and HSO leaves a strong acid catalytic site that may further favor the oxidative dehydrogenation cross linking charring process and increase the char yield in the charring process. Moreover, during combustion, SiO2 may occur on the surface of a burning composite, creating a physical protective barrier on the surface of the material [37]. It is, however, noted that the time corresponding to peak heat release rate prolongs with the loading of HSO. The reason may be due to the fact that SiO2 acts as a protective barrier in addition to the intumescent shield and can limit the oxygen diffusion to the substrate or give a less disturbing low volatilization rate.
From Fig. 1, it is interesting to find that PP0–PP4 samples only have a single peak, whereas there are two HRR peaks for PP5, which has good flame retardancy (a lower HRR). Bourbigot et al. also found this phenomenon in exploring APP/MEL/PER intumescent systems [27]. The one HRR peak is easily understood because the sample is gradually burnt. In the second case, the first peak is assigned to the development of the intumescent protective char [19]. After the first peak, the HRR curve forms a plateau in some cases, in which the increase in HRR is suppressed because of the presence of the efficient protective char. The second peak is due to the destruction of the protective layer with a lot of combustible gases when the sample is continuously exposed to the heat, and the formation of a new protective char. Conclusion can be made from above results that only suitable ratio of HSO to IFRs is needed to form an efficient intumescent protective char, and thus results in a decrease in PHRR.
The primary parameter which was responsible for HRR of the samples filled with IFR is the mass loss rate (MLR) during combustion, which was significantly reduced compared with those values observed for the pure polymer. Figure 2 shows that the MLR decreased in the order of PP5 > PP4 > PP3 > PP2 > PP1 > PP0, this trend is the same as those of the HRR in the cone calorimeter (Fig. 1). These similarities indicate that the mechanism of the observed reduction in HRR and also in MLR depends mainly on the condensed phase process instead of the gas phase process.
Figure 3 shows the weight of the char residues. During combustion, SiO2 may occur on the surface of the burning composite creating a physical protective barrier on the surface of material [28, 29]. The physical process of the layers reassembling would act as a protective barrier in addition to the intumescent shield and can thus limit the oxygen diffusion to the substrate or give a less disturbing low volatilization rate. The more HSO is added, the more SiO2 will be formed on the surface of the char residue. So, with the addition of HSO increases, the mass loss decreases.
Figure 4 presents the THR for all samples. The slope of THR curve can be assumed as representative of fire spread [10]. From Fig. 4, we can also see that the THR is decreased by the HSO at the time between 0 and 500 s. It is very clear that the flame spread of samples PP0–PP5 has decreased at the beginning, and the flame spread of sample PP5 is comparatively the lowest. It is also suggested there is a synergistic effect of flame retardancy between IFR and HSO. However, the THR of the samples with HSO is high than that of PP1. This can be explained that HSO is flammable, and the combustion of HSO can produce heat.
Figures 5 and 6 show the CO and CO2 produced from PP and flame retardant PP under a heat flux of 35 kW/m2. The incomplete combustion of flame retardant composite systems can be seen in the CO production rate. Compared with pristine PP, the CO production rate of flame retardant systems is highly decreased throughout the whole range of fire in the experiments. Furthermore, with the addition of HSO, the CO production rate decreases. It is very interesting that the CO production rate increases with the addition of HSO (from PP1 to PP3). Then, it decreases with the addition of HSO (from PP3 to PP5). This phenomenon can be illustrated that HSO is easily flammable. So, with the addition of HSO, there are more combustible gases (PP2 and PP3) released than that of PP1. On the combustion process, there are char residue formed on the surface of PP2 and PP3, which is not effective to restraint the combustible gases. It would lead much production of carbon monoxide. However, with the addition of HSO further increasing (PP4 and PP5), there are condensed char residues formed on the surface on the samples, which can effectively barrier the combustible gases into the flame zone. Furthermore, the char residue became condensed with the addition of HSO (from PP3 to PP5; Fig. 8). Hence, the carbon monoxide decreases. It should be figure out that there is an abrupt transition in the curve of PP5, which has a different pattern compared with PP1–PP4. This can be the results that when the time is about 370 s, the structure of the char residue formed in the cone calorimeter test broken, and a lot of combustible gases released. Then, much more carbon monoxide for PP5 produced than others.
The CO2 production rates of the flame retardant systems significantly decrease because the silicon-phosphate structure and a ceramic-like structure formed on the surface of the sample, which can prevent oxygen from being diffused into the flammable polymer.
Thermo gravimetric (TG) analysis
Figure 7 shows the TG curves of the pure PP and its flame retardant composites. It is clearly seen that all flame retardant PP composites decompose early in comparison with PP, which begins to decompose at about 260 °C. However, at a temperature higher than 270 °C, the FR PP composites are more thermally stable than PP. For example, PP almost decomposes completely at 400 °C, whereas the un-decomposed parts at the same temperature for PP1, PP2, PP3, PP4 and PP5 are 30.9%, 32.6%, 30.6%, 30.2% and 30.5%, respectively. The amount of residue of the FR PP composites at 600 °C is still higher than 14%. From 265 °C to 340 °C, the char residue of the sample with HSO is higher than PP1. The reason is that the silicon compound has higher thermal stability than APP/MEL/PER blends. From 430 °C to 570 °C, the char residue of the sample with the addition of HSO is similar with PP1. This can be interpreted that the PP1 sample without HSO can form more unstable char residue than other samples in this temperature range. It is interesting that when the temperature is raised to 600 °C, the char residues with HSO are higher than the PP1 sample. It can be interpreted that although PP1 sample produced more char residue than others from 430 °C to 570 °C, the structure is unstable, which can be further degrades with the temperature increasing. However, for the samples with HSO, there are some silicon-phosphate and ceramic-like structures formed on the surface of the samples, which can prevent the further degradation of the flammable polymer.
Digital photos of residues
Figure 8 are digital photos of residues of the series of samples. It can be seen that a more coherent and dense char can be formed with the addition of HSO. From the char structure, we can explain the combustion phenomenon of the flame retardant PP composites. The formation of the efficient char can prevent the heat transfer between the flame zone and the burning substrate, and thus protect the underlying materials from further burning and retard the pyrolysis of polymers. As a result, HRR values are strongly reduced, as shown in Fig. 1. The intumescent residue of PP5 was tighter, denser, and higher than any other residue, at the same time the residue of the samples with HSO were more dense and higher than PP1. The results are in accordance with the order in Fig. 1. This can be explained that the HSO can lead to the formation of ceramic-like material with a homogeneous surface which will protect the material throughout combustion and also to a mechanical reinforcement of the charred layer which would lead to a better accommodation of strains [36]. However, the micro composite may lead to the formation of an inhomogeneous and thus brittle surface material. The different char residues also contribute to the flame retardancy (FR) performance. For the above reasons the HRR of the samples with HSO is lower than that of PP1.
Conclusions
Flame retardant polypropylene composites were prepared by melt intercalation starting from APP, MEL, PER and HSO with the polymer matrix. Cone calorimeter experiments showed that a synergistic effect occurred when HSO and IFR are both present in polypropylene composites. HSO can decrease the heat release rate, mass loss, mass loss rate, and carbon dioxide, et al. However, a little of HSO can make the production of carbon monoxide increase. The HSO can enhance the high temperature stability of the PP/APP/MEL/PER/HSO samples. The morphological structure of char residue caused by HSO had been studied using digital photographs. It proves that the addition of HSO is capable of initiating a compact and homogeneous char on the surface, which turns out to be of most important for the flame retardant performance.
References
Sen AK, Mukheriee B, Bhattacharya AS, Sanghi LK, De PP, Bhowmick K (1991) J Appl Polym Sci 43:1674. doi:10.1002/app.1991.070430910
Shen H, Wang YH, Mai KC (2007) Thermochim Acta 457:27. doi:10.1016/j.tca.2007.02.023
Chen XL, Yu J, Guo SY (2007) J Appl Polym Sci 103:1978. doi:10.1002/app.24965
Plentz RS, Miotto M, Schneider EE, Forte MSMC, Mauler RS, Nachtigall SMB (2006) J Appl Polym Sci 101:1799. doi:10.1002/app.23558
Hong CH, Lee YB, Bae JW, Jho JY, Nam BU, Chang DH, Yoon SH, Lee KJ (2005) J Appl Polym Sci 97:2311. doi:10.1002/app.21776
Li B, Xu MJ (2006) Polym Degrad Stabil 91:1380. doi:10.1016/j.polymdegradstab.2005.07.020
Riva A, Camino G, Fomperie L, Amiqouet P (2003) Polym Degrad Stabil 82:341. doi:10.1016/S0141-3910(03)00191-5
Bourbigot S, Le Bras M (1995) Carbon 33:283. doi:10.1016/0008-6223(94)00131-I
Le Bras M, Bourbigot S, Christelle D (1996) Fire Mater 20:191 doi:10.1002/(SICI)1099-1018(199607)20:4<191::AID-FAM577>3.0.CO;2-S
Almeras X, Le Bras M, Hornsby P, Bourbigot S (2003) Polym Degrad Stabil 82:325. doi:10.1016/S0141-3910(03)00187-3
Almeras X, Le Bras M, Poutch F, Bourbigot S, Marosi G, Anna P (2003) Macromol Symp 198:435. doi:10.1002/masy.200350837
Bourbigot S, Le Bras M, Sophie D, Maryline R (2004) Macromol Mater Eng 289:499. doi:10.1002/mame.200400007
Bourbigot S, Duquesne S (2007) J Mater Chem 17:2283. doi:10.1039/b702511d
Chen YH, Wang Q (2006) Polym Degrad Stabil 91:2003. doi:10.1016/j.polymdegradstab.2006.02.006
Weil ED, Levchik S (2004) J Fire Sci 22:251. doi:10.1177/0734904104040546
Jahromi S, Gabrielse W, Braam A (2003) Polymer (Guildf) 44:25. doi:10.1016/S0032-3861(02)00686-9
Wu K, Song L, Wang ZZ, Hu Y (2008) Preparation and characterization of double shell microencapsulated ammonium polyphosphate and its flame retardance in polypropylene. J Polym Res. doi:10.1007/s10965-008-9228-9
Lv P, Wang ZZ, Hu Y, Yu MG (2009) J Polym Res 16:81. doi:10.1007/s10965-008-9205-3
Lv P, Wang ZZ, Hu KL, Fan WC (2005) Polym Degrad Stabil 90:523. doi:10.1016/j.polymdegradstab.2005.04.003
Wang XY, Li Y, Liao WW, Gu J, Li D (2008) Polym Adv Tech 19:1055. doi:10.1002/pat.1077
Camino G, Grassie N, McNeill IC (1978) J Polym Sci Polym Chem 16:95
Li B, Xu MJ (2006) Polym Degrad Stabil 91:1380. doi:10.1016/j.polymdegradstab.2005.07.020
Allen David W, Edwyn C (1994) Polym Degrad Stabil 45:399. doi:10.1016/0141-3910(94)90210-0
Hu XP, Li YL, Wang YZ (2004) Macromol Mater Eng 289:208. doi:10.1002/mame.200300189
Almeras X, Dabrowski F (2002) Polym Degrad Stabil 77:315. doi:10.1016/S0141-3910(02)00066-6
Demir H, Arkış E, Balköse D, Ülkü S (2005) Polym Degrad Stabil 89:478. doi:10.1016/j.polymdegradstab.2005.01.028
Bourbigot S, Le Bras M, Delobel R, Bréant P, Tremillon JM (1996) Polym Degrad Stabil 54:275. doi:10.1016/S0141-3910(96)00055-9
Tang Y, Hu Y, Wang SF, Gui Z, Chen ZY, Fan WC (2003) Polym Int 52:1396. doi:10.1002/pi.1270
Tang Y, Hu Y, Li BG, Liu L, Wang ZZ, Chen ZY, Fan WC (2004) J Polym Sci Polym Chem 42:6163. doi:10.1002/pola.20432
Ravadits I, Tóth A, Marosi G, Márton A, Szép A (2001) Polym Degrad Stabil 74:419. doi:10.1016/S0141-3910(01)00179-3
Marosi G, Márton A, Anna P, Bertalan G, Marosföi B, Szép A (2002) Polym Degrad Stabil 77:259. doi:10.1016/S0141-3910(02)00057-5
Estevão LRM, Le Bras M, Delobel R, Nascimento RSV (2005) Polym Degrad Stabil 88:444. doi:10.1016/j.polymdegradstab.2004.11.016
Wu Q, Qu BJ (2001) Polym Degrad Stabil 74:255. doi:10.1016/S0141-3910(01)00155-0
Yuan L, Qi W (2006) Polym Degrad Stabil 91:2513. doi:10.1016/j.polymdegradstab.2006.04.026
Li JM, Wilkie CA (1997) Polym Degrad Stabil 57:293. doi:10.1016/S0141-3910(97)00013-X
Zanetti M, Camino G, Thomann R, Mülhaupt R (2001) Polymer (Guildf) 42:4501. doi:10.1016/S0032-3861(00)00775-8
Zanetti M, Kashiwagi T, Falqui L, Camino G (2002) Chem Mater 14:881. doi:10.1021/cm011236k
Gilman JW, Jackson CL, Morgan AB, Harris R, Manias JE, Giannelis EP, Wuthenow M, Hilton D, Phillips SH (2000) Chem Mater 12:1866. doi:10.1021/cm0001760
Chiu SH, Wang WK (1998) Polymer (Guildf) 39:1951. doi:10.1016/S0032-3861(97)00492-8
Dabrowski F, Le Bras M, Cartier L, Bourbigot S (2001) J Fire Sci 19:219. doi:10.1106/WB1V-X0C6-G5EB-TC3J
Acknowledgement
The Doctoral Fund of QUST (No.0022355; No.0022394), the Foundation of State Key Laboratory of Fire Science (No.HZ2008-KF01) and the National Natural Science Foundation of China (No.50876048) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, X., Jiao, C. Synergistic effects of hydroxy silicone oil on intumescent flame retardant polypropylene system. J Polym Res 16, 537–543 (2009). https://doi.org/10.1007/s10965-008-9257-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-008-9257-4