Abstract
Graft and crosslinked polydimethylsiloxane (PDMS)-chitosan copolymers were prepared through the reaction between mono and difunctional glycidoxypropyl-terminated PDMSs and chitosan. The transformation of amino groups of chitosan through the reaction with epoxy groups was confirmed by FT-IR and 13C cross-polarization (CP) magic-angle spinning (MAS)-NMR analysis. Chitosan-based materials modified with about 40% and 60% hydrophobic polydimethylsiloxane were obtained, respectively. As proved by wide angle X-ray analysis, the crystallinity of chitosan was strongly decreased through the incorporation of PDMS sequences. However, both graft and crosslinked copolymers still present a partial crystalline structure. Their X-ray patterns are not only different as compared to chitosan but also as compared to each other. For the graft copolymer, three diffraction peaks were observed at 2θ = 8.4°, 11.2° and 21.2°, indicating the formation of a new partially crystalline phase and the modification of the interplanar distances for the phases similar to chitosan. The crosslinked copolymer is even less crystalline, the peak around 2θ = 20° being strongly decreased. Different thermal behaviour of siloxane modified chitosan was registered for graft and crosslinked copolymers; the graft sample is less stable than chitosan, while the crosslinked copolymer showed an intermediate stability between chitosan and polydimethylsiloxane precursors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Last years, much attention has been focused on the use of natural polymers in respect of environmental pollution. Among natural resources, chitin—obtained from the protective cuticle of crustaceans, the exoskeletons of insects and the cell walls of some fungi and microorganisms—as well as chitosan—derived by deacetylation of chitin—have been intensively studied due to their unique properties such as biocompatibility, non-toxicity, and antimicrobial activity. Chitosan has been used in detection of metal ion traces and in recovering the heavy metals from sea water or wastes [1–3]. Nowadays, the bonding of metal cations to chitosan continues to attract the interest of scientists [4–7] and the complexes were evaluated as antioxidants [8] or catalysts [9, 10]. Chitosan was also used in other applications such as biotechnology, pharmaceutics, cosmetics, agriculture, food science and textile industry [11–13]. However, some unsatisfactory mechanical properties, such as severe shrinkage, deformation after drying, low solubility in acidic media, and compressibility at high operating pressure, limit its application and processing convenience [14, 15]. Therefore, special attention has been paid to the chemical modification of chitosan.
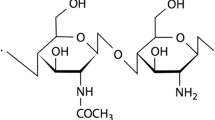
Chitosan (CS), an unbranched cationic biopolymer, has reactive functional groups which allow further chemical modification, i.e., amino or amido groups at C-2 positions as well as both primary and secondary hydroxyl groups at C-6, and C-3 positions, respectively. Crosslinking and graft copolymerization are well known methods for the modification of chitosan and represent convenient and effective ways for improving the physical and mechanical properties for practical uses. Various reagents, including epoxy compounds, glutaraldehyde [16, 17], etc. have been used as crosslinking agents for chitosan.
On the other hand, the introduction of siloxane moieties, especially polydimethylsiloxane (PDMS), into biomaterials can increase the oxygen permeability and/or the biocompatibility [18]. The high free volume of siloxane polymers as compared to the hydrocarbon polymers explains their solubility in low polar solvents and high diffusion coefficient for gases [19]. Moreover, it was already demonstrated that hardness and brittleness of chitosan—which limit its use, for instance, as wound healing materials [20]—is strongly modified through the incorporation of elastomeric PDMS (glass transition temperature around −120 °C). PDMS-CS graft copolymers [21] and interpenetrating networks containing poly(vinyl alcohol)-PDMS copolymer and chitosan components [22] were synthesized and the swelling of the resulted hydrogels was evaluated. Organosilica-CS crosslinked nanospheres [23] or membranes [24] were also prepared by crosslinking CS with γ-glycidoxypropyltrimethoxysilane.
This paper deals with synthesis of CS-PDMS graft and crosslinked copolymers by reacting mono or difunctional epoxy terminated PDMSs with CS. The microstructure of CS-PDMS copolymers induced by the grafting or crosslinking of CS was followed through wide angle X-ray diffraction. The synthesized copolymers were further proved to undergo complexation of metal cations [25].
Experimental
Materials
Low molecular weight CS with a degree of deacetylation of 75–85% was used as received (Aldrich). The precise value of the deacetylation degree was found to be about 80%, as determined from nitrogen content of the sample (8.23%). The intrinsic viscosity of CS sample was determined in 0.25 M acetic acid/0.25 M sodium acetate aqueous solution using an Ubbelohde viscometer kept in a constant temperature bath at 25 °C. The viscosity molecular weight was found to be approximately 380 kDa using the Mark–Houwink–Sakurada equation (MHS), [η] = K × M α, where [η] is the intrinsic viscosity and the empirical constants for the MHS equation were K = 1.81 × 10−3 (cm3/g), α = 0.93 [26–28]. Octamethylcyclotetrasiloxane (D4, 98%; Fluka), 1,1,3,3,-tetramethyldisiloxane (TMDS, 97%; Fluka), pentamethyldisiloxane (PMDS, 97%; Aldrich), sulfonated styrene-divinylbenzene ion exchange resin (Vionit CS 34C, Romanian product; exchange capacity = 4.2 meq/g, porosity = 39.42%, granulation = 0.4 ÷ 0.65 mm, specific surface = 35 m2/g) and allyl glycidyl ether (AGE, 99%; Aldrich) were used as received. Hexachloroplatinic acid monohydrate (Aldrich) was used as 2% by weight solution in isopropanol. Toluene was distilled under nitrogen on sodium wire before use.
Measurements
Infrared spectra (FT-IR) were obtained by using a Nicolet 60 SX FT-IR under dry air, at room temperature, on KBr pellets, in the range of 4,000–400 cm−1.
1H-NMR spectra were registered on a Bruker Avance 400 spectrometer in CDCl3. 13C cross-polarization (CP) magic-angle spinning (MAS)-NMR spectra were performed on a Bruker 125 MHz NMR 13C {1H} spectrometer.
X-ray diffraction patterns were recorded on a Rigaku K max-r Ax diffractometer with scanning scope of 5–40°, scanning speed of 4°/min, using Cu Kα radiation.
Themogravimetric analysis (TGA) was conducted with a Q-1500 D, MOM Budapest System at a heating rate of 10 °C/min under air atmosphere.
Active hydrogen in H-PDMS and epoxy group content in GP-PDMS precursors were determined by modified Zerewitinoff method [29] and from the integral ratio of the specific protons in 1H-NMR spectra, respectively.
Nitrogen content was obtained on a Perkin Elmer elemental analyser. Silicon content in PDMS-CS copolymers was established gravimetrically by sample decomposition with concentrated H2SO4, according to ref. [30].
Calculation:
where R: mass of SiO2 residue resulted by combustion (g); a: sample mass (g).
Synthesis of active hydrogen functionalized polydimethylsiloxanes (H-PDMS)
A monofunctional hydro-terminated polydimethylsiloxane (H-PDMS1) with predicted molecular weight was prepared by equilibration of D4 with PMDS as end-blocker (43.6 g D4/7.42 g PMDS) in the presence of 2.5% by weight dried Vionit CS 34 C catalyst under stirring for 2.5 h at 90 °C, according to a procedure previously described [31]. Cyclic compounds and unreacted chain stoppers were removed by distillation at 160 °C and 20 mmHg. The same procedure was used for the preparation of a difunctional hydro-terminated polydimethylsiloxane (H-PDMS2) in the presence of TMDS as end-blocker (46.6 g D4/13.7 g TMDS).
IR: (KBr), cm−1, 2,960–2,700 (CH2, CH3); 2,120 (Si–H); 1,260 (Si–CH3); 1,100–1,000 (Si–O–Si); 841 and 800 (Si–CH3).
1H-NMR: (CDCl3), δ (ppm), 0.06–0.11 (Si (CH3)2); 0.08–0.10 (CH3Si H); 4.74 (Si–H).
Synthesis of glycidoxypropyl terminated polydimethylsiloxane (GP-PDMSs)
GP-PDMSs were synthesized by the hydrosilation of allyl glycidyl ether with H-PDMSs in toluene, in the presence of hexachloroplatinic acid 2% by weight solution in isopropanol, according to a procedure previously described [32]. The complete conversion of Si–H groups to Si–C linkages was monitored by the IR spectra of the reaction mixture, through the disappearance of Si–H characteristic band at 2,120 cm−1. As confirmed by 1H-NMR spectra of GP-PDMSs, the hydrosilation reaction yielded mainly the β-addition products.
FT-IR: cm−1, 2,960–2,900 (CH2, CH3); 1,260, 845, 800 (Si–CH3); 1,024–1,092 (CH2–O–CH2 and Si–O–Si); 905 (epoxy).

The properties of siloxane precursors are presented in Table 1.
Synthesis of polydimethylsiloxane modified chitosan (CS-PDMS)
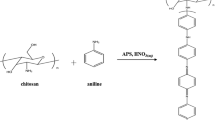
CS-PDMS graft and crosslinked copolymers were obtained by addition reaction between amine groups of chitosan and epoxy groups of polydimethylsiloxane (epoxy/amine groups = 1.4 molar ratio). The method is outlined as follows: 3 g chitosan were dissolved in 180 ml 0.5 M acetic acid aqueous solution (pH = 3). The solution was stirred at room temperature for 24 h, filtered to remove the insoluble material, and then bathed in an ultrasonic bath for 20 min. After standing for 24 h, the solution was added to 28.77 g GP-PDMS1 and 0.2 ml isopropyl alcohol. The reaction mixture was stirred for 24 h at 70 °C. The modified chitosan does not separate from solution. The solution was extracted in a funnel with diethyl ether to remove any unreacted polysiloxane (about 58% from initial amount). The solution was then poured into a glass mould and dried at 40 °C under vacuum up to constant weight to give 15 g of CS-PDMS1 (total yield, 42%). CS-PDMS2 crosslinked crosslinked copolymer was obtained by the same procedure starting from the difunctional GP-PDMS2 (epoxy/amine = 0.7 molar ratio). The unreacted GP-PDMS2 recovered by extraction was about 39%.
Results and discussion
CS-PDMS graft and crosslinked copolymers
CS-PDMS copolymers were prepared according to Scheme 1 by reacting mono or difunctional GP-PDMSs with CS. It is well known that the primary amine groups quickly undergo nucleophilic substitution with epoxy rings. In a first step, secondary amino groups are formed; they further undergo a second substitution yielding tertiary amines [32]. The reaction of amine groups of CS with epoxy rings linked to siloxane chains is quite difficult due to the very different solubility parameters of the mentioned polymers. In fact, the reaction takes place in a heterogeneous system, at the interface between CS aqueous solution and oil siloxane phase. After 24 h of reaction at 70 °C, only part of the siloxane polymer was linked to CS. However, in these reaction conditions, the yield of polysiloxane bonding to chitosan was as high as 42% or 61% for mono- and difunctional GP-PDMSs, respectively. The proportion of siloxane in CS-PDMS copolymers was established by silicon analysis and the fractions of nonreacted (f 1), bonded to PDMS chain (f 2) and acetylated CS structural units (f 3) were calculated (Table 2).
IR and NMR analysis
The structure of CS-PDMS copolymers was proved by FT-IR and 13C-NMR analysis.
The IR spectrum of modified chitosan (Fig. 1 shows the characteristic absorptions of CS-PDMS2 sample as typical example) presents the characteristic absorption of both precursors. A strongly diminished absorption band (large band centred at around 3,429 cm−1) associated to OH and NH2 groups in CS was evidenced in both CS-PDMS copolymers. The νC–O–C, νsim C–OH, and νas C–OH bands appearing at 1,157, 1,087, 1,026 cm−1 in CS are superposed on νSi–O–Si strong band in CS-PDMSs. CS-PDMSs IR spectra also contain Si–CH3 characteristic bands at 1,258 and 804 cm−1. The bands associated to partially acetylated CS centred at 1,650 (νC–O) and 1,596 cm−1 (νN–H) are enlarged between 1,654 and 1,550 cm−1 after modification of CS with PDMS.
Figure 2 presents for comparison the 13C-CP MAS NMR spectra of CS and CS-PDMS2. The spectrum of CS-PDMS2 copolymer shows the characteristic peaks of CS but they are broadened as compared to pure CS. The presence of siloxane sequences is evidenced in CS-PDMS spectrum by the peaks at δ, ppm, 0.0–0.8 (Si–CH3), 14.3 (Si–CH2), 25.5 (–CH2–C H 2–CH2–), 50.2 (CH2–NH–), 63.1 (–CH–OH), 71.5 and 73.7 (–CH2–CH2–O–CH2).
X-ray analysis of CS-PDMS copolymers
X-ray spectra of CS, CS-PDMS1 and CS-PDMS2 are shown in Fig. 3. The great intramolecular hydrogen bonding leads to chitosan crystalline character that distinguished it from other carbohydrate polymers [33]. As shown in Fig. 3, the XRD pattern of chitosan exhibits its characteristic crystalline peaks at 2θ = 10.2° and 19°. X-ray diffraction of CS-PDMS copolymers indicated that the bonding of PDMS to chitosan chains decreases the crystallinity. However CS-PDMS1 and CS-PDMS2 still presents a partial crystalline structure. It is interesting to notice that the graft and crosslinked copolymers present different X-ray patterns not only as compared to CS but also as compared to each other. For CS-PDMS1, three diffraction peaks were observed at 2θ = 8.4, 11.2 and 21.2°, indicating the formation of a new crystalline phase and the modification of the interplanar distances for the phases similar to CS. CS-PDMS2 is even less crystalline, the peak around 2θ = 20° being strongly decreased.
The above observations are also exemplified by the interplanar distances (d; Table 3) calculated using the Bragg’s equation λ = 2dsinθ, where θ is the diffraction position and λ is the wavelength.
Thermal behaviour of CS-PDMS copolymers
The thermal stability of graft and crosslinked copolymers was evaluated by thermogravimetric analysis and compared with CS, and epoxy-terminated PDMS precursors. As seen from Fig. 4, the most stable product is the epoxy-terminated PDMS that presents a weight loss of about 18% up to 345 °C and 65% up to 515 °C. Chitosan sample is less stable than PDMS. Up to about 340 °C it is loosing about 50% of the sample weight and at 750 °C it is practically completely decomposed. CS-PDMS2 crosslinked copolymer showed an intermediate behavior between CS and PDMS precursors. The weight loss of about 7% registered up to 150 °C could be attributed to the traces of solvent and to still adsorbed water. The compound starts to decompose at 150 °C, loosing a total of about 39% and 85% up to 360 and 750 °C, respectively, the remaning residue being mainly silicon dioxide. It is interesting to notice that CS-PDMS1 graft copolymer presents a quite strange decomposition curve. The copolymer is less stable than chitosan, showing a more rapid decomposition up to about 550 °C. Moreover, it is totally decomposed up to 750 °C. No silicon dioxide is formed as a result of the decomposition of siloxane sequence. This feature is probably due to a synergistic effect of chitosan on the thermal decomposition of siloxane component, with the formation of volatile silicon containing cyclic and linear oligomer species, which occurred during heating of graft copolymer but was not observed for the crosslinked copolymer.
Conclusions
Chitosan was successfully modified with polydimethylsiloxane by the ring opening reaction of epoxy groups linked to siloxane chain with primary amino groups of chitosan. Due to the large difference between solubility parameters of chitosan and polysiloxane, the reaction occurred in heterogeneous conditions. However, a quite large amount of siloxane moieties were linked to the biopolymer. The modified chitosan presents lower crystallinity as compared to the pure polymer. The thermal stability was found to be dependent on the copolymer structure; the graft copolymer is less stable than both chitosan and epoxy-terminated polysiloxane precursors, while the crosslinked copolymer shows an intermediate behaviour. The known ability of chitosan to bind metal cations is maintained after the modification with polysiloxane, as proved elsewhere [25].
Reference
Onsøyen E, Skaugrud Ø (1990) J Chem Techn Biotechn 49:395–404
Kurada K, Sannan T, Iwakura T (1979) J Appl Polym Sci 23(2):511–515
Varma AJ, Deshpande SV, Kennedy JF (2004) Carbohydr Polym 55:77–93
Braier NC, Jishi RA (2000) J Molec Struct (Theochem) 499:51–55
Vold IMN, Vårum KM, Guibal E, Smidsrød O (2003) Carbohydr Polym 54:471–477
Sreenivasan K (1996) Polym Degradat Stability 52:85–87
Monteiro OAC Jr, Airoldi C (2005) J Colloid Interf Sci 282:32–37
Yin X, Zhang X, Lin Q, Feng Y, Yu W, Zhang Q (2004) Arkivoc (ix):66–78
Kramareva NV, Finashina ED, Kucherov AV, Kustov LM (2003) Kinet Catal 44(6):792–800
Xue L, Zhou DJ, Tang L, Ji XF, Huang MY, Jiang YY (2004) React Funct Polym 58:117–121
Muzzarelli RAA, Jeuniaux C, Gooday GW (1986) Chitin in nature and technology. Plenum, New York, p 7981
Li Q, Dunn ET, Grandmaison EW, Goosen MFA (1997) Applications and properties of chitosan. Technomic, Lancaster, pp 3–29
Hudson SM, Smith C, Kaplan DL (1998) Polysaccharide: chitin and chitosan: chemistry and technology of their use as structural materials, In: Biopolymers from renewable resources (ed). Springer, New York, pp 96–118
Rabea EI, Badaway MET, Stevens CV, Smagghe G, Meysteurbaut W (2003) Biomacromolecules 4:1457–1465
Kawamura Y, Mitsuhashi M, Tanibe H, Yoshida H (1993) Ind Eng Chem Res 32:386–91
Sashiwa H, Shigemasa Y (1999) Carbohydr Polym 39:127–138
Jameela SR, Jayakrishnan A (1995) Biomaterials 16:769–775
Nahrup JS, Gao ZM, Sakr A (2004) Int J Pharm 270:199–208
Noll W (1968) Chemistry and technology of silicones. Academic, New York, p 437
Khan T, Peh KK, Chng HS (2003) J Pharm Pharmaceut Sci 6(1):20–26
Kim Y, Kim SJ, Shin MS, Lee YM, Shin DI, Kim SI (2002) J Appl Polym Sci 85:2661–2666
Shin MS, Kim SI, Kim IY, Kim NG, Song CG, Kim SJ (2002) J Appl Polym Sci 84:2591–2596
Fei B, Lu H, Xin JH (2006) Polymer 47:947–950
Liu YL, Su YH, Lai JY (2004) Polymer 45:6831–6837
Enescu D, Hamciuc V, Timpu D, Harabagiu V, Simionescu BC (2007) J Optoelectr Adv Mater (in press)
Kassai MR, Arul J, Charlet G (2000) J Polym Sci Part B Polym Phys 38:2591–2598
Liu L, Li Y, Li Y, Fang Y-E (2004) Carbohydr Polym 57:97–100
Galed G, Miralles B, Panos I, Santiago A, Heras A (2005) Carbohydr Polym 62:316–320
McHard JA (1959) In: G. M. Kline (ed), Analytical chemistry of polymers, Vol. 13, Intersicence, New York, Part. I, Chap. XIV, p 374
Shaefer WE (1937) Ind Eng Chem Anal Ed 9:449
Giurgiu D, Hamciuc V, Butuc E, Cozan V, Stoleriu A, Marcu M, Ionescu C (1996) J Appl Polym Sci 59(10):1507–1514
Harabagiu V, Pinteala M, Cotzur C, Holerca MN, Ropot M (1995) J Macromol Sci Part A: Pure Appl Chem A32(8&9):1641–1648
Roberts GAF (1992) Chitin chemistry. Macmillian, London, p 230
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Enescu, D., Hamciuc, V., Pricop, L. et al. Polydimethylsiloxane-modified chitosan I. Synthesis and structural characterisation of graft and crosslinked copolymers. J Polym Res 16, 73–80 (2009). https://doi.org/10.1007/s10965-008-9204-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10965-008-9204-4