Abstract
Chitosan-graft-polyaniline (Chi-g-PANI) copolymer was successfully synthesized via the chemical oxidative polymerization method. The influence of various synthesis conditions, including the weight ratio of chitosan/aniline, amount of ammonium persulfate (APS), concentration of nitric acid ([HNO3]) and synthesis temperature, on grafting % and electrical conductivity of Chi-g-PANI copolymer was investigated. The synthesized Chi-g-PANI copolymer was characterized by Fourier transform infrared spectroscopy (FTIR), ultraviolet–visible spectrophotometer (UV–Vis), 13C solid-state nuclear magnetic resonance (NMR) spectroscopy, X-ray diffractometer (XRD), field-emittance scanning electron microscope (FE-SEM) and a four-point measurement method. The optimum synthesis condition was identified as the weight ratio of chitosan/aniline = 2, amount of APS = 0.49 g, [HNO3] = 0.1 M and synthesis temperature at 25 °C. The grafting % and electrical conductivity of the Chi-g-PANI copolymer at the optimized conditions were 92% and 8 × 10–3 S/cm, respectively. Results of FTIR and 13C solid-state NMR showed that PANI was successfully grafted onto the molecular chains of chitosan. UV–Vis analysis confirmed that the electronic transition behavior of Chi-g-PANI copolymer was similar to that of PANI. Meanwhile, XRD results revealed the crystallinity of chitosan was destroyed by PANI during copolymerization. Furthermore, FE-SEM results demonstrated that the morphology of the Chi-g-PANI copolymer was obviously different from that of chitosan or PANI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chitosan is a natural nontoxic biopolymer derived by deacetylation of chitin, a natural polysaccharide widely found in the shells of crustaceans and insects [1]. Chitosan is a linear polysaccharide composed of randomly distributed β-(1 → 4) -linked D-glucosamine (deacetylated unit) and N-acetyl-D-glucosamine (acetylated unit). The degree of deacetylation (%DD) in commercial chitosans ranges from 60 to 100%. In recent years, chitosan is widely used in wastewater treatments [2], separation membranes [3, 4], drug delivery systems [5] and biosensors [6, 7] due to its unique biodegradable, biocompatible nonirritant [8], good film-forming properties, high mechanical strength and adhesion [9].
Intrinsically conducting polymers (ICP), such as polyaniline, polythiophene, and polypyrrole, are commonly used in scientific and industrial research in a variety of applications. Currently, ICPs have become an efficient alternative to inorganic conductors in many practical applications [10], such as rechargeable batteries [11], sensors [12, 13] and photovoltaics [14]. Compared with other ICPs, polyaniline (PANI) has been attracting attention because it is easy to be synthesized and exhibits a wide range of conductivity, low operational voltage, as well as good environmental, thermal, and chemical stability. Meanwhile, this compound contains attractive electrochemical, electronic, optical and electro-optical properties. However, a major problem to limit PANI’s utilization is its poor mechanical properties and processability due to its insoluble nature in common solvents.
Graft copolymerization is a significant and common way to enhance the compatibility of the chitosan with synthetic polymers [15]. Recently, in order to expand the application of chitosan and polyaniline, Chi-g-PANI copolymer has been widely studied. The chemical grafting of polyaniline onto the molecular chain of chitosan was reported by Yang et al. [16]. Their results showed that the electronic behavior of copolymer film was similar to that of PANI film. Desbrieres et al. [17] adopted chemical oxidative polymerization to synthesize Chi-g-PANI copolymer and then blended with chitosan to obtain electrically conductive hydrogel using glutaraldehyde as a crosslinking agent. The results showed the hydrogel formed was a superabsorbent hydrogel and the swelling was reversible. Tiwari et al. demonstrated chemical and biosensor potential applications of Chi-g-PANI copolymer [18]. Ma et al. developed a new series of in-situ forming antibacterial conductive degradable hydrogels using quaternized chitosan (QCS) grafted polyaniline with oxidized dextran as a crosslinker. This research opens the way to fabricate in situ forming antibacterial and electroactive degradable hydrogels as a new class of bioactive scaffolds for tissue regeneration applications [19]. Yazdi et al. synthesized Chi-g-PANI copolymer and used it as a sorbent for the preconcentration of phthalate esters in dispersive solid-phase extraction. By coupling dispersive solid-phase extraction with high performance liquid chromatography and response surface methodology (central composite design), a reliable, sensitive and cost-effective method for simultaneous determination of phthalate esters was developed [20]. Shukla et al. reported the extractive potentiometric sensing of lead ions over a chemically functionalized ternary nanocomposite of nickel oxide intercalated chitosan grafted polyaniline (NiO-in-CHIT-g-PANI) prepared by the in situ chemical polymerization and composite formation technique under optimized conditions [21].
In this paper, the chemical oxidative polymerization method was adopted to synthesize PANI on chitosan to form a conductive Chi-g-PANI copolymer which showed relatively good biocompatibility and electrical conductivity. Meanwhile, in order to find out the optimum synthesis condition, the effect of synthesis conditions on grafting % and conductivity was systematically studied including the weight ratio of chitosan/aniline, amount of APS, concentration of [HNO3] and synthesis temperature. Furthermore, a conductive Chi-g-PANI hydrogel was also prepared by chemical crosslinking of the conductive Chi-g-PANI solution in this study.
Experimental
Materials
Chitosan was purchased from CHARMING & BEAUTY Company (Taiwan). The average molecular weight and degrees of deacetylation (%DD) were about 200,000 g/mol and 95%, respectively. Aniline and ammonium persulfate (APS) were purchased from Merck (Germany). N-Methylpyrrolidone (NMP) was purchased from Shimadzu (Japan). Glutaraldehyde (GA) was purchased from Aldrich Chemical and used as a chemical crosslinking reagent.
Synthesis of PANI
0.2 g of aniline monomer (0.0021 mol) was added in beaker containing 200 ml of 0.1 M HNO3 under constantly stirring until it was completely dissolved. 0.49 g APS (0.0021 mol) was added into this mixture for polymerization. After 4-h reaction, the darkish PANI solution was filtered and then washed with RO water until the pH of filtrate reached about 6 ~ 7.
Synthesis of Chi-g-PANI copolymer
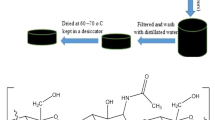
The synthesis procedure of Chi-g-PANI copolymer was described by Desbrieres et al. [17]. A controlled amount of chitosan (0.2, 0.4, 0.6, 0.8 and 1.0 g, respectively) was dissolved in 0.1 MHNO3 solution (200 ml) while constantly stirring until fully dissolved to form a chitosan solution. Then, 0.2 g of aniline (0.0021 mol) was added to the chitosan solution. After that, the controlled amount of APS (0.245, 0.368, 0.490, 0.615 and 0.735 g, respectively) was added into the chitosan solution. After 4 h reaction, darkish green Chi-g-PANI copolymer dispersion was precipitated out via using NaOH(aq). Then, the excess ethanol was added into the dispersion under constantly stirring. The precipitated material was filtered and then washed with NMP and RO water to remove free PANIs, unreacted monomers and the initiator. After that, the resulted material was dissolved in 0.1 M HNO3 solution (200 ml) to form the doped Chi-g-PANI copolymer. Furthermore, the solution containing Chi-g-PANI copolymer was precipitated with excess ethanol. Finally, the synthesized product was filtered and dried at the room temperature. In the study, the grafting % and conductivity of the synthesized product was discussed by varying the synthesis conditions (weight ratio of chitosan/aniline, amount of APS, concentration of HNO3 solution and reaction temperature) to determine the optimum synthesis conditions.
Preparation of Chi-g-PANI copolymer conductive hydrogel
10 ml of Chi-g-PANI copolymer solution was put in a 25-ml glass bottle. Then, the crosslinking reagent (glutaraldehyde) was slowly added under constantly stirring. The final concentration of glutaraldehyde in the Chi-g-PANI copolymer solution was controlled as 3 wt%. And then, the mixture was placed at room temperature (∼25 °C) for 24 h.
Characterization
Fourier transform infrared (FTIR) analysis
The chemical structures of chitosan, PANI and Chi-g-PANI copolymer were identified using a Fourier transform infrared (FTIR) spectrometer (Spectrum One; Perkin Elmer, USA). The samples and dehydrated KBr (weight ratio of sample/KBr = 1/99) were grounded together into fine powders, and then the homogeneous mixtures were pressed to form pellets for analysis. The wavenumber was ranged from 600 to 4000 cm−1 with the scanning rate as 64/sec.
13C solid-state NMR analysis
The chemical structures of chitosan and Chi-g-PANI copolymer were identified using a 13C solid-state nuclear magnetic resonance (NMR) spectrometer (BRUKER-NMR 400 MHz). The weight of samples used was in the range from 0.3 to 0.5 g.
DC electrical conductivity test
0.1 g ES PANI was weighed and then pressed under 3.0 × 105 psi for 2 min at room temperature. The four-point measurement method was used to examine the electrical conductivity σ (S/cm) of the Chi-g-PANI copolymer at room temperature.
Determination of grafting %
The following was the formula to calculate the percentage of grafting. It was similar to the method described by Desbrieres et al. [17].
mChi-g-PANI: the weight of Chi-g-PANI copolymer, mANI: the weight of aniline monomer, mchitosan: the weight of chitosan.
UV–Vis analysis
The chitosan, synthesized PANI, and synthesized Chi-g-PANI copolymer were dissolved in 0.1 M nitric acid at room temperature, respectively. Then, optical absorbance of the PANDB solution in the wavelength range of 250–900 nm was determined using an ultraviolet–visible spectrophotometer (UV–Vis; Shimadzu, model UV-2401 PC).
XRD analysis
The samples were studied at room temperature by using a Rigaku’s X-ray diffractometer. The X-ray source was nickel-filtered CuKα radiation (40 kV, 30 mA). The scanning angle 2θ was varied in the range from 5° to 60° at a speed of 1°/min.
Field-emittance scanning electron microscope (FE-SEM)
The surface morphology of chitosan, PANI and Chi-g-PANI copolymer was investigated by scanning electron microscopy at 10 kV with a JEOL (Japan) JSM-840A scanning microscope. All specimens were coated with a conductive layer of sputtered platinum.
Results and discussion
Determination of optimal synthesis conditions
Figure 1 shows the synthesis reaction of electrically conductive Chi-g-PANI copolymer via chemical oxidative radical graft polymerization. It indicated that polyaniline grafting occurred at NH2 groups of chitosan [22]. Figure 2 shows the relationship of grafting % and conductivity vs. weight ratio of chitosan/aniline when the synthesis conditions were fixed at 0.49 g APS (0.0021 mol), 0.1 M HNO3, 25 °C synthesis temperature and 4 h reaction time. Results indicated when the weight ratio of chitosan/aniline was in the range from 1 to 2, the grafting % was increased from 86 to 92%. If the weight ratio of chitosan/aniline was beyond 2, no evident change in grafting % was observed; even the ratio was increased up to 4.
This was because in the polymerization system, part of the aniline polymerized into pure PANI, and part of the aniline polymerized into chitosan-g-PANI. When the amount of chitosan increased, the probability of synthesizing chitosan-g-PANI also increased. But as the amount of chitosan was higher, the viscosity of the reaction solution also increased. This slowed down the diffusion rate of aniline and hindered the polymerization reaction of aniline. Therefore, when the weight ratio of chitosan/aniline was beyond 2, no evident change in grafting% was observed.
Based upon this observation, the optimum weight ratio of chitosan/aniline was maintained as 2 in the following studies. Meanwhile, the maximum conductivity (0.036 S/cm) of Chi-g-PANI copolymer was reached when the weight ratio of chitosan/aniline (0.2/0.2 g) was set as 1. When the weight ratio of chitosan/aniline was further increased, the conductivity of the synthesized Chi-g-PANI copolymer was decreased. This was due to the increase in the viscosity of the reaction medium causing hindrance in the normal reaction when the amount of chitosan was increased. Therefore, the conductivity of the synthesized Chi-g-PANI copolymer was decreased.
Figure 3 illustrates the influence of APS (as an initiator) on the grafting % and conductivity of Chi-g-PANI copolymer. The other reaction conditions were controlled at weight ratio of chitosan/aniline = 2, 0.1 M HNO3, synthesis temperature of 25 °C, and reaction time of 4 h. Results showed that grafting % and conductivity were increased when the amount of APS was increased from 0.245 to 0.49 g. The optimized grafting % (92%) and conductivity (0.0081 S/cm) of Chi-g-PANI copolymer were obtained while 0.49 g APS was used. Reason for this observation was when the amount of APS increases, it simultaneously activates the -NH2 group of chitosan, which causes more aniline monomers to polymerize onto the chitosan backbone. Thus, both grafting % and conductivity were increased. However, when the adding amount of APS exceeds 0.49 g, the grafting % and conductivity of synthesized Chi-g-PANI copolymer do not improve significantly. Therefore, the optimum amount of APS as 0.49 g (0.0021 mol), i.e. the mole ratio of aniline/APS = 1, was identified and further used in this study.
Figure 4 shows the influence of concentration of HNO3 in the range from 0.05 to 0.15 M on the grafting % and conductivity of Chi-g-PANI copolymer. The amount of APS, weight ratio of chitosan/aniline, reaction temperature and time were fixed as 0.49 g, 2, 25 °C and 4 h, respectively. It was observed that both grafting % and conductivity increase with increasing the concentration of HNO3. It may be due to the higher degree of protonation of the aniline monomer and acceleration of the propagation of aniline, which can generate more PANI ion radicals [18]. Therefore, the optimum concentration of HNO3 was identified as 0.1 M in this study.
Figure 5 illustrates the influence of synthesis temperature on the grafting % and conductivity of Chi-g-PANI copolymer. Three reaction temperatures (0, 25, 50 °C) were studied while keeping other synthesis conditions constantly; weight ratio of chitosan/aniline = 2, amount of APS = 0.49 g, concentration of nitric acid = 0.1 M and reaction time 4 h. Maximum grafting % and conductivity of Chi-g-PANI copolymer were obtained at 25 °C. The result was attributed to the viscosity of the reaction solution at 0 °C was higher than 25 °C and 50 °C. It affected the diffusion efficiency of aniline in the reaction solution and reduced the probability of PANI grafted on chitosan. Therefore, the grafting % and conductivity of chitosan-g-PANI both decreased at 0 °C. At 25 °C, the viscosity of the reaction solution was lower than at 0 °C, and the probability of PANI grafted on chitosan was effectively increased. It resulted in both the grafting % and conductivity of chitosan-g-PANI significantly increased. At 50 °C, the viscosity of the reaction solution was the lowest. Although the diffusion efficiency of aniline in the reaction solution increased significantly, it also increased more aniline to synthesize pure PANI or oligomers. Therefore, both the grafting % and conductivity of chitosan-g-PANI were lower than those synthesized at 25 °C. The experimental results showed that the optimal temperature for the synthesis of chitosan-g-PANI was 25 °C in this study.
Characterization of Chi-g-PANI copolymer
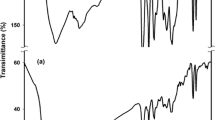
Figure 6 shows the FTIR spectra of chitosan, PANI and Chi-g-PANI copolymer, respectively. Figure 6a shows FTIR spectrum of chitosan. The characteristic peaks appearing in the range from 3600 cm−1 to 3000 cm−1 were due to the stretching vibrations of –OH and –NH groups of chitosan. Meanwhile, the characteristic peak appearing at 1636 cm−1 was due to C = O stretching vibration of carbonyl group of the amide group CONHR. In addition, the characteristic peak appearing at 1541 cm−1 was attributed to –NH bending vibration of chitosan. Furthermore, the characteristic peaks appearing at 1068 and 1024 cm−1 were due to –CO stretching vibration of sugar rings of chitosan. Figure 6b exhibits FTIR spectrum of PANI, and the characteristic peaks appearing at 1578 and 1494 cm−1 were due to the stretching vibrations of N = Q = N ring and N–B–N ring, respectively. The characteristic peak appearing at 1303 cm−1 was attributed to C–N stretching vibration of the secondary amine in the PANI backbone. Also, the characteristic peak appearing at 829 cm−1 was due to aromatic C–H bending vibration band due to the 1,4-disubstituted benzene ring.
Figure 6c shows FTIR spectrum of Chi-g-PANI copolymer. It can be observed that the characteristic peak of C = O stretching vibration of chitosan was overlapped with that of N = Q = N ring stretching vibration of PANI to form a broad band from 1680 to 1560 cm−1. Meanwhile, the characteristic peak of stretching vibration of N–B–N ring was shifted to the higher wavenumber from 1494 to 1506 cm−1 due to the conjugated length of PANI in Chi-g-PANI copolymer was shorter than that of pure PNAI. Moreover, Fig. 6c shows characteristic peaks of chitosan (1068 and 1024 cm−1) as well as PANI (1303 and 829 cm−1) [18]. Hence, FTIR results indicated that PANI was grafting onto chitosan backbone successfully.
Figure 7 shows the 13C NMR spectra of chitosan and Chi-g-PANI copolymer. Solid-state 13C NMR was an efficient method to confirm the graft structure of the products. The characteristic peak of chitosan can be interpreted as δ = 58 ppm (C2), 62 ppm (C6), 76 ppm (C5, C3), 84 ppm (C4), and 106 ppm (C1). For Chi-g-PANI copolymer, the chemical shift values of 58 and 62 ppm were corresponded to C2 and C6, 73 and 76 ppm corresponded to C3, C4, and C5, and 99 ppm corresponded to C1, respectively, in the chitosan structure. The chemical shift value of 126 ppm corresponds to C7, which was protonated aromatic carbons of PANI, in the Chi-g-PANI copolymer [23]. The solid-state 13C NMR spectra illustrated that the Chi-g-PANI was synthesized successfully.
Figure 8 demonstrates the UV–Vis absorption spectra of chitosan, PANI and Chi-g-PANI copolymer. One characteristic absorption peak appearing in the chitosan solution, at around 310 nm, was attributed to the glucopyranose components of chitosan as shown in Fig. 8a. Three characteristic absorption peaks were found in the PANI solution at around 350, 450, and 800 nm as shown in Fig. 8b. The absorption peak appearing at around 350 nm was ascribed to π–π* transition of the benzenoid rings, while the peaks appearing at around 450 and 800 nm were attributed to polaron–π* transition and excition transition of quinoid rings, respectively. Figure 8c shows the UV–Vis spectrum of Chi-g-PANI copolymer. It showed a broad absorption band around 300–360 nm due to overlapping of glucopyranose components of chitosan and π–π ∗ transition of benzenoid rings of grafted PANI, while the peaks appearing at 450 and 700 nm were attributed to polaron–π* transition and excition transition of quinoid rings, respectively [18]. Although the peak resulted from excition transition of quinoid rings of ES-type PANI was shifted from 800 to 700 nm due to the conjugated length of PANI in Chi-g-PANI copolymer was shorter than that of pure PNAI, the UV–Vis results indicate that the electronic state of Chi-g-PANI copolymer was similar to that of ES-type PANI. Furthermore, the characteristic peaks of glucopyranose and PANI were significantly observed and this result supported the grafting of PANI onto chitosan backbone to form electrically conductive Chi-g-PANI copolymer.
Figure 9 shows the XRD spectra of chitosan, PANI and Chi-g-PANI copolymer. The XRD spectrum of chitosan showed two peaks around 12° and 21° due to the existence of both amorphous and crystalline regions, respectively [24]. The XRD spectrum of PANI revealed one peak at 23° due to the crystalline structure of PANI. Meanwhile, the spectrum of the Chi-g-PANI copolymer contained one peak around 18–23° due to the overlap of crystalline peak of chitosan and amorphous peak of PANI. The peak at 12° was disappeared due to the grafted polyaniline. The XRDs results indicated that the crystallinity of chitosan was destroyed by PANI due to the chemical oxidative radical grafting polymerization.
Figure 10 illustrates FE-SEM images of the surface morphology of chitosan, PANI and Chi-g-PANI copolymer. The pure chitosan showed a smooth and dense surface with no peculiar morphology; as in Fig. 10a. The surface morphology of PANI was in the form of granules or rods and was stacked and agglomerated as shown in Fig. 10b. Moreover, these granules or rods were also mostly in nanometer size. However, the surface morphology of the Chi-g-PANI copolymer was fibrous with a diameter of about 10 to 30 nm, which was significantly different from the surface morphology of chitosan and PANI. This was because PANI was graft polymerized on the molecular chain of chitosan, resulting in a fibrous surface morphology as shown in Fig. 10c. The FE-SEM micrographs indicated that the surface morphology of Chi-g-PANI copolymer was different from those of pure chitosan and PANI.
Figure 11a shows the photograph of Chi-g-PANI copolymer solution before adding glutaraldehyde (crosslinking reagent). The Chi-g-PANI copolymer solution was very fluidity. However, after glutaraldehyde was added into Chi-g-PANI copolymer solution, a chemical crosslinking reaction occurred, thus forming a Chi-g-PANI conductive hydrogel, and the conductivity was about 0.01 S/cm as shown in Fig. 11b. The resulted Chi-g-PANI conductive hydrogel contained good biocompatibility, processability with improved solubility, mechanical strength and controlled electrical properties [18]. The conductive Chi-g-PANI copolymer solution and hydrogel have potential to apply as biosensors, biomaterials, drug delivery, bioactuators, self-healing and antibacterial materials [25].
Conclusions
The conductive Chi-g-PANI copolymer was synthesized by a chemical oxidative radical graft polymerization. The influence of synthesis conditions on electrical conductivity and grafting % of Chi-g-PANI copolymers was systematically studied. FTIR, UV–Vis and 13C solid-state NMR results showed that conductive PANI were successfully grafted onto the chitosan backbone. The results revealed that the optimum synthesis conditions were weight ratio of chitosan/aniline = 2, amount of APS = 0.49 g, concentration of HNO3 = 0.1 M, and synthesis temperature = 25 °C. Meanwhile, the electrical conductivity and grafting % were 8 × 10–3 S/cm and 92% at the optimum condition. The Chi-g-PANI copolymer was an intrinsic conductive copolymer with pH switching behavior like PANI. The electrical conductivity of the resulted copolymer was depended on the extent of grafting and pH of the material. After a suitable chemical crosslinking reaction, a Chi-g-PANI conductive hydrogel can be obtained. At the same time, because of the excellent properties of Chi-g-PANI, the application of conductive Chi-g-PANI copolymer solution or hydrogel will be able to widely develop in the future.
References
Li B, Wang X, Chen R, Huang FW, Xie G (2008) Antibacterial activity of chitosan solution against Xanthomonas pathogenic bacteria isolated from Euphorbia pulcherrima. Carbohydr Polym 72:287–292
Tabriz A, Alvi MAUR, Khan Niazi MB, Batool M, Bhatti MF, Khan AL, Khan AU, Jamil T, Ahmad NM (2019) Quaternized trimethyl functionalized chitosan based antifungal membranes for drinking water treatment. Carbohydr Polym 207:17–25
Varghese JG, Karuppannan RS, Kariduraganavar MY (2010) Development of hybrid membranes using chitosan and silica precursors for pervaporation separation of water + isopropanol mixtures. J Chem Eng Data 55:2084–2092
Unlu D, Hilmioglu ND (2018) Pervaporation catalytic membrane reactor application over functional chitosan membrane. J Membr Sci 559:138–147
Panos I, Acosta N, Heras A (2008) New drug delivery systems based on chitosan. Curr Drug Discov Technol 5:333–341
Yavuz AG, Uygun A, Bhethanabotla VR (2010) Preparation of substituted polyaniline/chitosan composites by in situ electropolymerization and their application to glucose sensing. Carbohydr Polym 81:712–719
Kang X, Wang J, Wu H, Aksay IA, Liu J, Lin Y (2009) Glucose Oxidase–graphene–chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens Bioelectron 25:901–905
Gupta KC, Jabrail FH (2007) Glutaraldehyde cross-linked chitosan microspheres for controlled release of centchroman. Carbohydr Res 342:2244–2252
Mucha M, Wankowicz K, Balcerzak J (2007) Analysis of water adsorption on chitosan and its blends with hydroxypropylcellulose. e-Polym 16:1–10
Hrehorova E, Bliznyuk VN, Pud AA, Shevchenko VV, Fatyeyeva KY (2007) Electrical properties and fractal behavior of polyurethane elastomer/polyaniline composites under mechanical deformation. Polymer 48:4429–4437
He BL, Dong B, Wang W, Li HL (2009) Performance of polyaniline/multi-walled carbon nanotubes composites as cathode for rechargeable lithium batteries. Mater Chem Phys 114:371–375
Khan R, Dhayal M (2009) Chitosan/polyaniline hybrid conducting biopolymer base impedimetric immunosensor to detect Ochratoxin-A. Biosens Bioelectron 24:1700–1705
Yavuz AG, Uygun A, Bhethanabotla VR (2009) Substituted polyaniline/chitosan composites: synthesis and characterization. Carbohydr Polym 75:448–453
Yavuz AG, Uygun A, Can HK (2011) The effect of synthesis media on the properties of substituted polyaniline/chitosan composites. Carbohydr Polym 346:2063–2069
Kumar D, Gihar S, Shrivash MK, Kumar P, Kundu PP (2020) A review on the synthesis of graft copolymers of chitosan and their potential applications. Int J Biol Macromol 163:2097–2112
Yang S, Tirmizi SA, Burns A (1989) Chitaline materials: Soluble Chitosan-polyaniline copolymers and their conductive doped forms. Synth Met 32:191–200
Marcasuza P, Reynaud S, Desbrieres J (2010) Chitosan-graft-polyaniline-based hydrogels: elaboration and properties. Biomacromol 11:1684–1691
Tiwari A, Sing V (2007) Synthesis and characterization of electrical conducting chitosan-graft-polyaniline. eXPRESS Polym Lett 1:308–317
Zhao X, Li P, Guo BL, Ma PX (2015) Antibacterial and conductive injectable hydrogels based on quaternized chitosan-graft-polyaniline/oxidized dextran for tissue engineering. Acta Biomater 26:236–248
Razavi N, Yazdi AS (2017) New application of chitosan-grafted polyaniline in dispersive solid-phase extraction for the separation and determination of phthalate esters in milk using high-performance liquid chromatography. J Sep Sci 40:1739–1746
Kushwaha CS, Shukla SK (2020) Potentiometric extractive sensing of lead ions over a nickel oxide intercalated chitosan-grafted-polyaniline composite. Dalton Trans 49:13862–13871
Ibrahim MS, Abd El-Mageed HR, Abd El-Salam HM (2020) Density functional theory calculations on the grafting copolymerization of 2-substituted aniline onto chitosan. Polym Bull 77:6391–6407
Mathew R, Mattes BR, Espe MP (2002) A solid state NMR characterization of cross-linked polyaniline powder. Synth Met 131:141–147
Wan Y, Wu H, Yu AX, Wen DJ (2006) Biodegradable polylactide/chitosan blend membranes. Biomacromol 7:1362–1372
Min JH, Patel M, Koh WG (2018) Incorporation of conductive materials into hydrogels for tissue engineering applications. Polymers 10:1078
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, CH., Wu, HT., Mao, CF. et al. Conductive chitosan-graft-polyaniline copolymer: synthesis and characterization. Polym. Bull. 79, 6259–6273 (2022). https://doi.org/10.1007/s00289-021-03818-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-021-03818-3